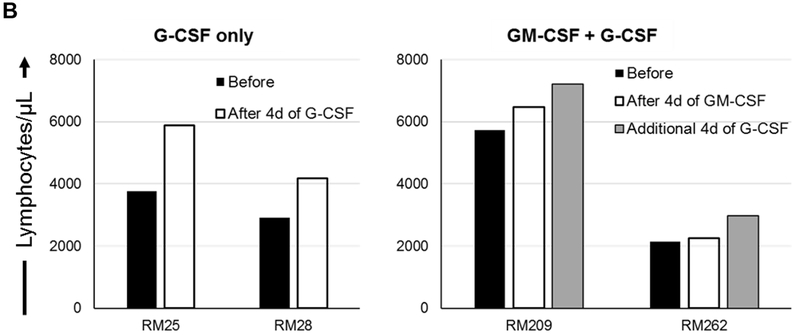
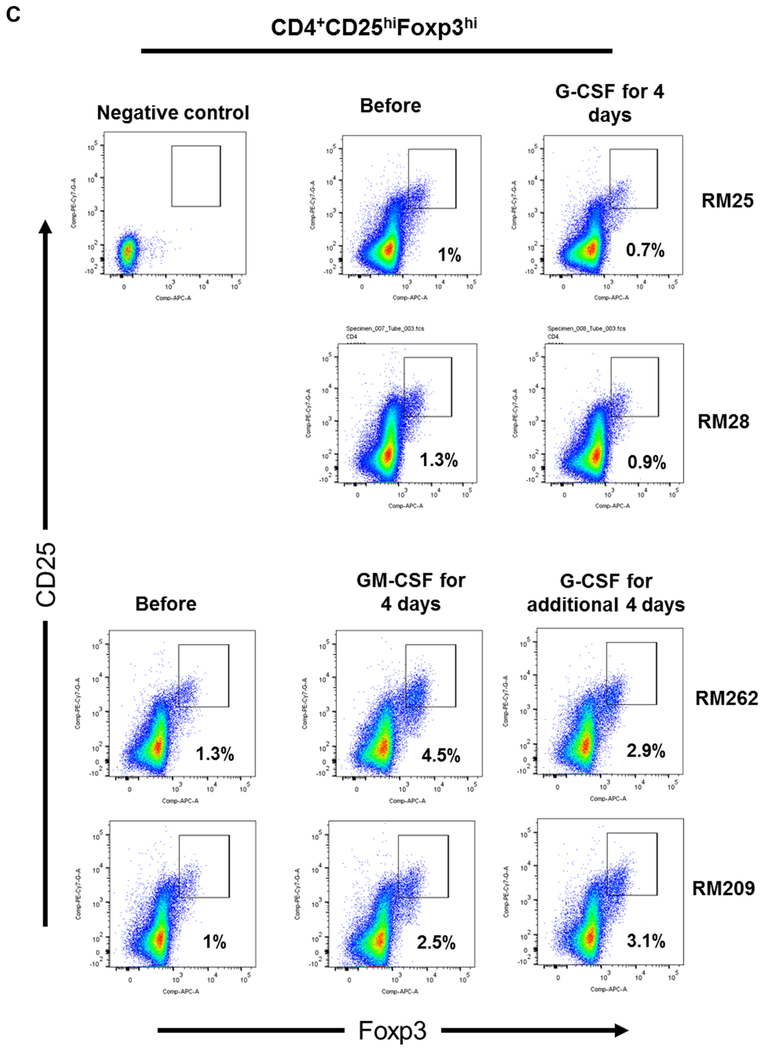
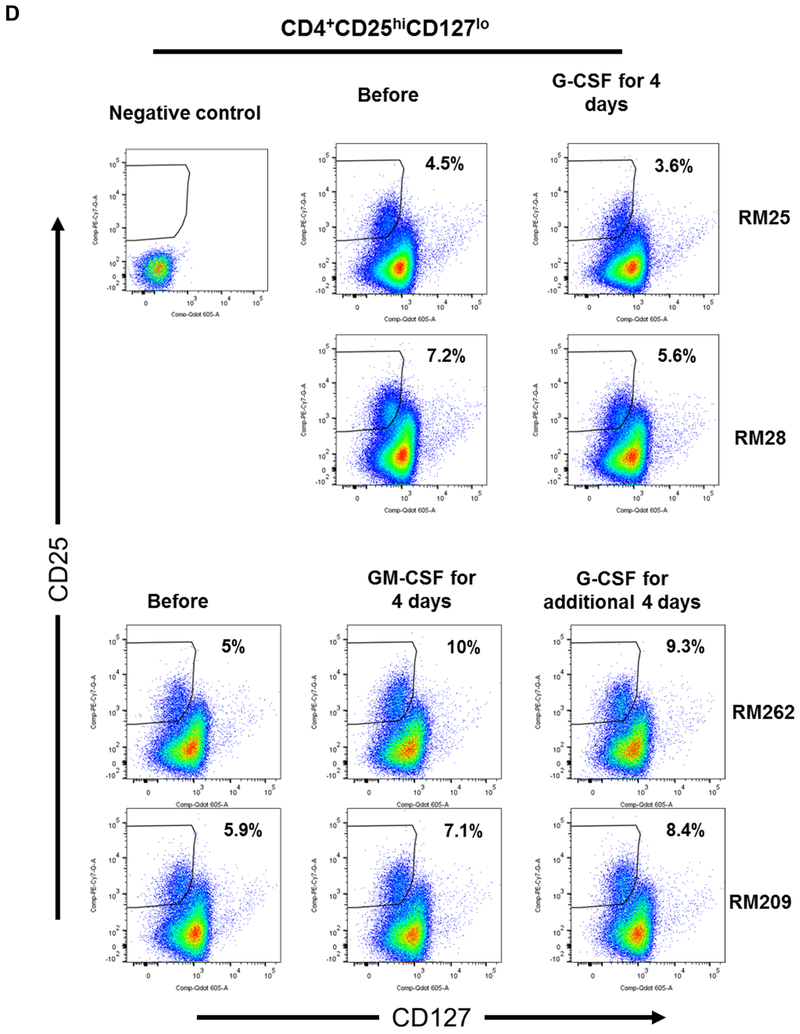
FIGURE 1. Impact of G-CSF, GM-CSF, or combined GM-CSF/G-CSF administration on the percentages of peripheral blood Treg in rhesus monkeys.
(A) Monkeys received subcutaneous injections of either recombinant (r) human G-CSF (Neupogen 10 μg/kg/day) only for four days (n=2), or r human GM-CSF (Leukine; 10 μg/kg/day) for four days followed by r human G-CSF (Neupogen 10 μg/kg/day) for an additional four days (n=2). (B) Absolute numbers of lymphocytes in peripheral blood before and after G-CSF, GM-CSF, or GM-CSF/G-CSF administration. (C) Percentages of CD4+CD25hiFoxp3hi Treg in peripheral blood before and after G-CSF, GM-CSF, or GM-CSF/G-CSF administration. (D) Percentages of CD4+CD25hiCD127lo Treg before and after G-CSF, GM-CSF, and GM-CSF/G-CSF administration. Peripheral blood samples were collected before, after G-CSF administration, after GM-CSF administration, and after combined GM-CSF/G-CSF administration. Top left dot plots indicate isotype (negative) controls.