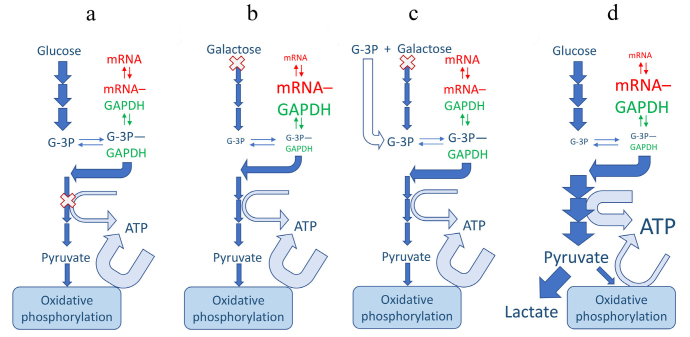
Fig. 1.
Mechanisms of regulation of ARE-mRNA abundance by glycolytic flux. Enzymes may be regulated by fluxes only indirectly through changes in metabolite abundances. Accordingly, the moonlighting mRNA-binding function of GAPDH is regulated by abundance of its substrates, G-3P and pyridine nucleotides. GAPDH, mRNA and a skeleton scheme of glycolytic pathway are shown in green, red and blue, respectively; basal lactate production is inconsequential and omitted for simplicity (a-c). Width of the arrows depicts the magnitude of the fluxes and the size of the font depicts the abundance of metabolites. (X) Indicates the restriction point in the pathway; under normal conditions (a) the restriction points are ATP-producing enzymes that are suppressed by competition from mitochondrial ATP production. Galactose shares most of classical glycolytic pathway but is slow to enter it (b and c). This leads to GAPDH binding, and depleting, of ARE-mRNAs, including IFNγ (b), the process reversible by exogenous G-3P (c) [27]. A hypothetical glycolytic switch scenario (d) could lead to a decreased IFNγ mRNA (and protein), in apparent discrepancy with observed IFNγ production. This discrepancy would require additional research to resolve.