Abstract
Klotho is a membrane-bound protein acting as an obligatory coreceptor for fibroblast growth factor 23 (FGF23) in the kidney and parathyroid glands. The extracellular portion of its molecule may be cleaved and released into the blood and produces multiple endocrine effects. Klotho exerts anti-inflammatory and antioxidative activities that may explain its ageing suppression effects evidenced in mice; it also modulates mineral metabolism and FGF23 activities and limits their negative impact on cardiovascular system. Clinical studies have found that circulating Klotho is associated with myocardial hypertrophy, coronary artery disease and stroke and may also be involved in the pathogenesis of salt-sensitive hypertension with a mechanism sustained by inflammatory cytokines. As a consequence, patients maintaining high serum levels of Klotho not only show decreased cardiovascular mortality but also non-cardiovascular mortality. Klotho genetic polymorphisms may influence these clinical relationships and predict cardiovascular risk; rs9536314 was the polymorphism most frequently involved in these associations. These findings suggest that Klotho and its genetic polymorphisms may represent a bridge between inflammation, salt sensitivity, hypertension and mortality. This may be particularly relevant in patients with chronic kidney disease who have decreased Klotho levels in tissues and blood.
Keywords: ageing, all-cause mortality, cardiovascular mortality, FGF23, Klotho
Patients with chronic kidney disease (CKD) are characterized by an increased risk of both cardiovascular and all-cause mortality that are 8.8 and 8.1 times higher, respectively, in dialysis patients than the general population [1, 2]. Alterations of mineral metabolism typical of CKD, hyperphosphataemia, secondary hyperparathyroidism, fibroblast growth factor 23 (FGF23) hyperproduction and 1,25-dihydroxyvitamin D (1,25(OH)2D) deficiency have been identified as non-classical risk factors for cardiovascular mortality and morbidity [3]. Among these variables, FGF23 acquired a negative prognostic value because of its association with cardiomyocyte hypertrophy and vascular calcification observed in clinical and laboratory studies [4]. Serum FGF23 was also associated with non-cardiovascular mortality in CKD patients, and this association was attributed to the pro-inflammatory effects of FGF23 [5, 6]. The activity of FGF23 in the parathyroid glands and kidney is mediated by its cell membrane receptor(FGFR) and its obligatory membrane-bound coreceptor Klotho, necessary for the organ-specific activity of FGF23 [7]. Studies in mice identified Klotho as a novel ageing suppressor [8]. Accordingly, studies in humans showed that Klotho may be involved in a variety of features typical of ageing, such as vascular calcification, atherosclerosis, osteomalacia, osteoporosis, insulin resistance, cardiovascular and metabolic alterations and resistance to oxidative stress [8, 9].
This issue of CKJ includes a study by Cambray et al. [10] reporting the association of genetic single-nucleotide polymorphisms (SNPs) of the Klotho gene (KL) with non-cardiovascular mortality in a group of Spanish patients with CKD Stages 3–5d. This article provides us the opportunity to focus on the role of Klotho in human physiology and physiopathology. Klotho proteins are a family of proteins including α-, β- and γ-Klotho. In this article, we consider only α-Klotho (hereafter Klotho), which is the only circulating form of Klotho proteins and was the subject of the study of Cambray et al. [10].
KLOTHO BIOLOGY
Klotho is a 140-kDa protein (1.012 amino acids) encoded by the KL gene (13q13.1), having a high homology with β-glucosidases [8]. Two different Klotho protein types may be detectable in humans: the first type is a full-length single-pass transmembrane protein located in the cell plasma membrane that consists of an N-terminal signal sequence, a large extracellular domain (130 kDa, 950 amino acids) and a short intracellular domain. The other one is soluble Klotho, circulating in blood and including both a cleaved soluble and a secreted Klotho. Cleaved soluble Klotho is generated by cleavage of the entire extracellular portion of membrane-bound Klotho by α- and β-secretases and the activity of which is stimulated by insulin, while the secreted Klotho results from an alternative transcript splicing at exon 3 [11]. Membrane-bound Klotho is mainly expressed in kidney tubules, mostly in distal segments, parathyroid glands, choroid plexus, the sinoatrial node and minimally in bones and cartilage [12]. As a consequence of this distribution, a large proportion of soluble Klotho is released into the systemic circulation by secretase-mediated shedding in kidney tubular cells. Klotho expression in the kidney is enhanced by 1,25(OH)2D and blunted by aldosterone and angiotensin II, as well as by albuminuria and inflammation mediators [13, 14]. Soluble Klotho acts as a hormone having its targets in tissues not expressing Klotho [15] and regulating ion reabsorption through autocrine and paracrine effects (Table 1) [11, 15]. Serum levels of Klotho decrease with ageing [16] and in patients with CKD because of a reduction of the nephron mass and 1,25(OH)2D synthesis. For these same reasons, membrane-bound Klotho expression is also downregulated in the kidney and parathyroid glands of CKD patients [17, 18].
Table 1.
Activities of soluble Klotho
| Anti-ageing activities |
| Inhibition of insulin/IGF-1 signalling pathway |
| Regulation of calcium and phosphate metabolism |
| Suppression of oxidative stress |
| Suppression of inflammation |
| Inhibition of tissue fibrosis after inflammatory disorders |
| Regulation of blood pressure response to NaCl load |
| Protective activity against myocardial hypertrophy |
| Protection against coronary artery disease |
| Protection against stroke |
| Decrease in blood pressure |
| Prevention of artery wall calcification |
| Anti-osteoporosis effects |
| Anti-cancer activity |
| Anti-obesity activity |
KLOTHO IN MINERAL AND ION METABOLISM
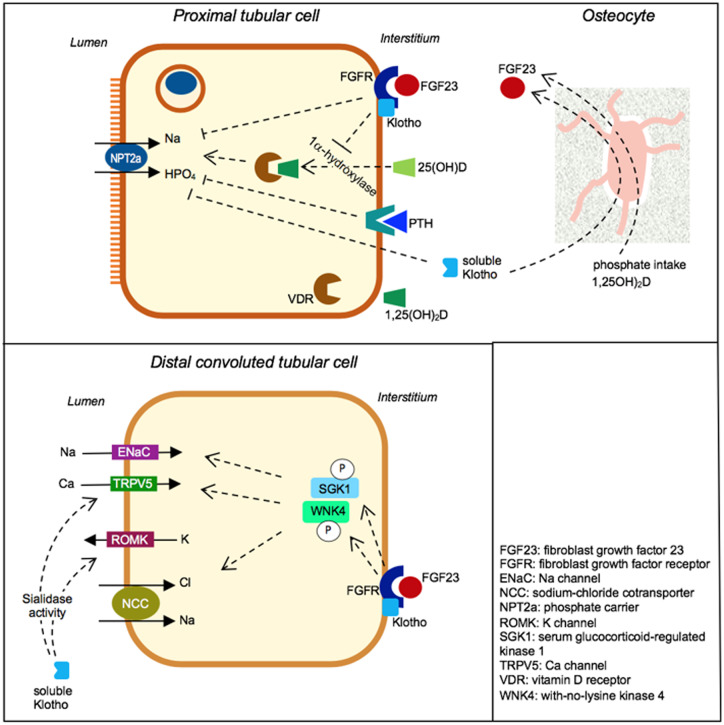
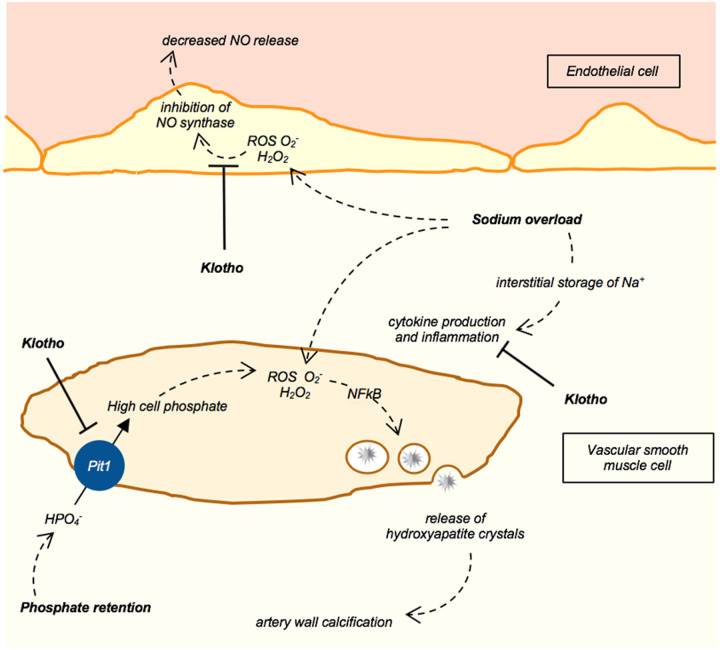
In the proximal tubule, Klotho acts in concert with FGF23 (Figure 1). The complex FGF23–Klotho–FGFR causes inhibition of phosphate reabsorption through internalization of the inward sodium–phosphate cotransporter type IIa (NPT2a) [11, 15]. This complex can also downregulate 1,25(OH)2D synthesis through the inhibition of renal 1-α-hydroxylase activity. In distal convoluted tubular cells (Figure 1), the cooperative activity of FGF23 and Klotho induces the phosphorylation of kinases (SGK1 and WNK4) upregulating the expression of epithelial sodium channel(ENaC), sodium chloride cotransporter (NCC) and transient receptor potential calcium channel(TRPV5), and thus sodium and calcium reabsorption [15, 19, 20]. Soluble Klotho may also directly activate rectifier potassium channel 1 (ROMK) and TRPV5 channels for calcium transport through the apical membrane of distal convoluted tubular cells [19, 20]. The effects on ROMK and TRPV5 might be due to the sialidase activity of soluble Klotho that may influence the glycosylation state of these channels (Figure 1), thus preventing their internalization and upregulating their membrane expression in tubular cells [15]. The hypophosphataemic rickets developed by patients carrying the KL gene translocation suggest that soluble Klotho may directly downregulate expression of the phosphate carrier NPT2a in the proximal tubule [21]. Klotho activities in phosphate metabolism also include the inhibition of phosphate transporter 1 (PiT1; encoded by SLC20A1) activity expressed in vascular smooth muscle cells (VSMCs), tubular cells and osteoblasts. Phosphate entry through PiT1 induces transformation of VSMCs in osteoblast-like cells (Figure 2) that are able to produce bone matrix proteins and deposit hydroxyapatite crystals in the artery wall [22]. Through the inhibition of PiT1, Klotho may protect against the degenerative process of artery wall calcification and stiffening, whereas Klotho deficiency amplifies vascular calcification risk in CKD patients [22, 23]. Soluble Klotho may also sustain FGF23 production by osteocytes (Figure 1) and thus Klotho supports the hypophosphataemic effect of FGF23 and exerts a direct vascular intervention to protect against artery calcification risk [24].
FIGURE 1.
Effects of soluble Klotho and transmembrane Klotho in proximal and distal tubular cells. In proximal cells, transmembrane Klotho, included in a complex with FGF23 and FGFR, inhibits phosphate reabsorption and 1α-hydroxylation of 25-hydroxyvitamin D [25(OH)D]. In distal convoluted tubular cells, Klotho stimulates the phosphorylation of WNK4 and SGK1 and the expression of the sodium channel ENaC, potassium channel ROMK, NCC and calcium channel TRPV5. As a result, Klotho activates sodium and calcium reabsorption and potassium excretion through these carriers. Klotho sialidase activity was hypothesized to increase membrane expression of TRPV5 and ROMK in distal tubular cells. In addition, soluble Klotho increases FGF23 production in osteocytes. Arrows indicate activation and lines with a flat end indicate inhibition of the considered activity.
FIGURE 2.
Klotho may suppress inflammation and oxidative stress in VSMCs and endothelial cells. Phosphate entry in VSMCs through PiT1 carrier stimulates the production of reactive oxygen species such as superoxide () and hydrogen peroxide (H2O2). These compounds activate nuclear factor-κB signalling pathways and increase the expression of osteogenic factors in VSMCs that are stimulated to develop an osteoblast-like phenotype. Thus these cells can release membrane vesicles containing hydroxyapatite crystals and cause artery wall calcification. In addition to these mechanisms, sodium overload increases sodium content in the artery wall. This causes local inflammation and production of ROS and O2 in endothelial cells, impairing nitric oxide synthesis and its vasodilating activity.
KLOTHO AND THE RENIN–ANGIOTENSIN–ALDOSTERONE SYSTEM
In vitro and in vivo studies showed that blockade of the renin–angiotensin–aldosterone system (RAAS) may increase soluble Klotho levels [13, 15]. Valsartan treatment significantly increased mean soluble Klotho levels compared with amlodipine in diabetic patients [25]. By 16 weeks of age heterozygous Klotho-deficient mice (KL+/−) developed spontaneous and persistent elevation of blood pressure along with increased aldosterone plasma levels not coupled with an increase in renin and angiotensin II but due to the upregulation of the expression of enzymes involved in aldosterone synthesis [25]. Blockade of aldosterone actions by eplerenone reversed hypertension and attenuated the kidney damage [26]. This study further showed that upregulation of aldosterone levels was responsible for the inflammation seen in KL+/− mice because blockade of aldosterone receptors abolished leucocyte infiltration and cytokine releases in the kidney [26]. These studies suggested Klotho's role in the control of blood pressure.
KLOTHO, INFLAMMATION AND OXIDATIVE STRESS
Compelling evidence indicates the antioxidative and anti-apoptotic activities of Klotho protein and its role in preventing inflammation and fibrosis by the inhibition of insulin/insulin-like growth factor-1 (IGF-1) and transforming growth factor-β1 (TGF-β1) signalling pathways and suppression of the the release of pro-inflammatory cytokine interleukion 6 (IL-6) [27–29]. Inhibition of the insulin–IGF-1 signalling pathway and its downstream phosphatidylinositol 3-kinase (PI3K)–serine–threonine kinase (Akt) signalling pathway leads to activation of the forkhead box protein O transcription factors (FoxOs) and enhanced the expression of manganese superoxide dismutase, a superoxide neutralizer. FoxOs control genes involved in the cellular differentiation, growth and survival, cell cycle, glucose and lipid metabolism and detoxification of reactive oxygen species [27, 30]. Consequently, negative regulation of the PI3K–Akt pathway by Klotho results in an oxidative stress resistance that contributes to anti-ageing mechanisms, including the prevention of oxidative DNA damage [31]. Klotho deficiency causes CKD patients to lose protective mechanisms against oxidative stress and inflammation that predisposes them to cardiovascular events [32].
The fibrogenic effect of TGF-β1 was abolished by soluble Klotho in mice kidneys, as it was directly bound to type II TGF-β receptor and inhibited TGF-β1 binding [27]. Downregulation of the TGF-β1 pathway by Klotho was also observed in renal and myocardial tissues of angiotensin II–infused mice in which Klotho prevented angiotensin II–induced cardiac remodelling and dysfunction [28].
In primary human umbilical vascular endothelial cells and mononuclear cells from atherosclerotic patients, Klotho overexpression had a potent role in inhibiting IL-6 production, an important mechanism leading to protection of the cardiovascular system [29].
Pro-inflammatory cytokines such as TGF-β1 and tumuor necrosis factor-α(TNF-α) were observed to decrease renal expression of Klotho through epigenetic mechanisms in animal models with kidney injury. Albuminuria was also observed to decrease renal Klotho expression through epigenetic mechanisms, but it also decreased renal Klotho expression through the activation of interstitial inflammation [14, 33]. Findings in KL+/− mice proposed a relevant connection between inflammation and hypertension, as theCC chemokine receptor 2–mediated inflammatory process was involved in salt(NaCl)-sensitive hypertension developed in these mice [34]. Renal structural injury and functional impairment resulting from this process were sustained by inflammatory cytokine activation and cell infiltration upregulating SGK1-NCC signalling in the kidney in response to high salt loading. Hypertension was abolished by administration of a specific chemokine antagonist [34]. The combination of these data suggests that Klotho may be a crucial node in the link between sodium handling, blood pressure, RAAS, inflammation and ageing.
KL POLYMORPHISMS, CARDIOVASCULAR OUTCOME AND MORTALITY
Different polymorphisms in the KL gene were tested before the article by Cambray et al. [10]. The so-called KL-VS is a haplotype of the KL gene including six SNPs in a region of 800 bp in exon 2 and flanking sequence. These SNPs were in complete linkage disequilibrium and one of them, F352V (rs9536314 T>G), which causes amino acid substitution, was used to tag the KL-VS haplotype. The G allele was associated with lower human longevity [35] and was an independent risk factor for coronary artery disease in healthy siblings of patients with early-onset coronary artery disease [36] and withhigh-density lipoprotein cholesterol, systolic blood pressure, stroke and mortality in individuals ≥75 years of age after adjustment for traditional risk factors [37]. More recently, an Italian study associated F352V with salt-sensitive hypertension, as patients carrying the G allele were more represented among hypertensive patients who were salt-sensitive [38]. Carriers of the G allele also showed higher sodium excretion in Italian adolescent students, suggesting an early contribution of Klotho to sodium metabolism and blood pressure control [39].
Another intronic Klotho variant (rs577912, C>A) was linked to the 1-year risk of all-cause mortality in incident chronic haemodialysis patients. The CC genotype–associated increase of mortality risk was not detected in patients treated with active vitamin D, suggesting that this therapy could modify the risk of death. A study of the CC genotype in lymphoblastoid cell lines observed that it caused a 16–21% decrease in Klotho expression compared with the AA/AC genotype. [40]. Finally, the KL gene SNP G395A, located in the promoter region of the gene, was proposed as a risk factor of coronary artery disease in the Asian population [41].
KLOTHO, CARDIOVASCULAR OUTCOME AND MORTALITY
Non-genetic studies confirmed that Klotho deficiency is an independent predictor of cardiovascular and all-cause mortality in chronic haemodialysis patients and the elderly population [15, 42]. Experiments in laboratory animals indicated a direct protective effect of soluble Klotho on the cardiovascular system. Klotho-deficient mice exhibited calcification of the artery walls [8] and cardiac dysfunction, characterized by hypertrophy and fibrosis, while an increase in soluble Klotho alleviated cardiac remodelling induced by FGF23, a high-phosphate diet and CKD [43]. Elevated serum levels of FGF23 and reduced serum levels of soluble Klotho may contribute to uraemic cardiomyopathy in a synergistic manner. Klotho was shown to decrease FGF23 expression and cardiac remodelling in angiotensin II–infused mice with a pathway involving TGF-β1 expression [27, 28]. As already reported, Klotho is involved in the control of blood pressure, probably through its influence in sodium handling, inflammatory cytokine activation, tissue cell infiltration and response to salt [34, 38, 39]. In addition, Klotho gene delivery increased nitric oxide production and thus reduced blood pressure values and vascular endothelial dysfunction in rats with multiple atherosclerosis risk factors [44].
KLOTHO AND NON-CARDIOVASCULAR OUTCOMES
The study by Cambray et al. [10] genotyped a group of Spanish patients with CKD Stages 3–5d for 11 polymorphisms of the KL gene selected according to the findings of the National Observatory of Atherosclerosis in Nephrology study and analysed for the first time [10]. Three of these SNPs, rs562020 (A>G), rs2320762 (T>G) and rs2283368 (T>C), were associated with non-cardiovascular mortality. These SNPs were intronic and their effect on gene expression was not investigated, therefore Cambray et al. [10] did not identify a pathogenetic link between Klotho and this outcome. It is noteworthy that the patients’ mean age was lower in this study than in others in CKD [1–3], thus representing a useful condition to identify the role of Klotho in mortality before the development of cardiovascular disease.
Previous studies in haemodialysis patients associated all-cause mortality with low serum levels of Klotho and a minor allele at SNP rs577912, decreasing Klotho expression in lymphoblastoid cells [40, 42]. These findings could be explained with the anti-ageing activity of Klotho [14, 34]. The phenotype of transgenic mice carrying a loss-of-function mutation of the KL gene was characterized by a short lifespan, infertility, arteriosclerosis, skin atrophy, hypertension, atrophy of the thymus and gonads, cardiac hypertrophy and fibrosis, sarcopaenia, ectopic calcifications, osteoporosis and emphysema [8]. This complex phenotype, which may be overall defined as early ageing, was alleviated by Klotho overexpression with a mechanism involving insulin–IGF-1–PI3K signalling [9].
The renoprotective activity of Klotho was observed in animal models of acute kidney injury and CKD. Mice with glomerulonephritis carrying a Klotho transgene improved their survival and renal functions, decreased morphological lesions at both the tubular and glomerular level and reduced cytochrome c oxidase activity, β-galactosidase activity (a senescence-associated protein), mitochondrial DNA fragmentation, superoxide anion generation, lipid peroxidation and apoptosis [45]. The renoprotective effect of Klotho was also found in mice developing renal fibrosis after unilateral ureteral obstruction. Treatment of these mice with Klotho protein suppressed the process of fibrosis and prevented the epithelial–mesenchymal transition associated with fibrosis. Soluble Klotho also suppressed TGF-β1 signalling and WNT3 and WNT4 activity, which were inducers of the epithelial–mesenchymal transition [27, 28].
Soluble Klotho may protect against non-cardiovascular effects of FGF23, which may exert its noxious properties when serum Klotho is reduced [15]. The weight of FGF23 as a potential cause of death in CKD patients may be due to its pleiotropy, as it was associated with the risk of end-stage renal disease and acute kidney injury, bone fractures, severe infections and inflammation and even progression of cancer [6, 15].
Serum phosphate and phosphate intake, as food additives or phosphate-rich foods, were linked to all-cause mortality and could negatively influence health in CKD and non-CKD patients [46]. Klotho-deficient mice increased their serum levels of phosphate and developed ageing symptoms, including emphysema, which might be alleviated by feeding these mice a low-phosphate diet [15]. Phosphate was able to directly induce oxidative injury of the lungs through increased DNA oxidative stress and apoptosis, while Klotho exerted antioxidant effects protective of lung injury induced by phosphate, hyperoxia and acute Klotho deficiency [47].
INDICATIONS FOR THE NEXT FUTURE
This FGF23–Klotho system potentially contributes to the pathophysiology of multiple disorders in humans, including CKD, arteriosclerosis, cardiac hypertrophy, hypertension, diabetes, obesity and a few types of cancer. Interventions to activate the endocrine effects of soluble Klotho could represent a new approach for the treatment of these ageing-related disorders (Table 1). According to Hu et al. [48], the use of recombinant Klotho indicated that its deficiency is pathogenetic for CKD and that its replacement with recombinant Klotho may be a feasible, safe and efficacious therapy not only against the progression of acute CKD, but also against cardiac remodelling in post-acute kidney injury. Moreover, Klotho therapy may protect against phosphotoxic insults, correct oxidative stress and decrease organ inflammation and fibrosis. These effects may be healthy for many organs, but they appeared to be specifically beneficial for the lungs, for unclear reasons [15]. Oxidative stress and inflammation related to Klotho deficiency were recently associated with hypertension and albuminuria in diabetic mice and could be determined by phosphate retention in CKD [49]. The KL gene variants may be associated with salt-sensitive hypertension, which may represent an abnormal blood pressure response to sodium load in order to maintain normal plasma volume and sodium chloride balance mainly due to blunted renin–angiotensin, even though no difference in the RAAS was found according to genotype [38]. KL polymorphisms could cause alterations of vascular systems and inflammatory–immune responses, which may lead to the development of sodium sensitivity in young subjects and predispose to an inflammatory state in adulthood, predisposing elderly patients to cardiovascular risk [50]. Therefore salt sensitivity associated with Klotho pathways may be proposed as a bridge between cardiovascular and non-cardiovascular mortality. Several articles support the role of the anti-inflammatory effect of Klotho in the relationship between salt and inflammation [51]. Patients with unfavourable KL polymorphisms or low plasma levels of Klotho could develop a positive sodium balance over the course of years, leading to a chronic inflammatory condition and an increase in blood pressure values (Figure 2). This process could be of particular interest in CKD patients who progressively decrease their serum levels of Klotho earlier than subjects with normal renal function and could explain the increase in non-cardiovascular mortality in adulthood and the development of hypertension that contributes to cardiovascular mortality in the elderly. Thus the therapeutic activity of Klotho in CKD patients represents a fascinating and promising perspective for future studies.
CONFLICT OF INTEREST STATEMENT
The authors have declared no conflicts of interest. This article has not been published previously in whole or part.
REFERENCES
- 1. de Jager DJ, Grootendorst DC, Jager KJ et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 2009; 302: 1782–1789 [DOI] [PubMed] [Google Scholar]
- 2. Thompson S, James M, Wiebe N et al. Cause of death in patients with reduced kidney function. J Am Soc Nephrol 2015; 26: 2504–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van de Luijtgaarden MW, Jager KJ, Segelmark M et al. Trends in dialysis modality choice and related patient survival in the ERA-EDTA Registry over a 20-year period. Nephrol Dial Transplant 2016; 31: 120–128 [DOI] [PubMed] [Google Scholar]
- 4. Scialla JJ, Wolf M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol 2014; 10: 268–278 [DOI] [PubMed] [Google Scholar]
- 5. Singh S, Grabner A, Yanucil C et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 2016; 90: 985–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Czaya B, Faul C. FGF23 and inflammation – a vicious coalition in CKD. Kidney Int 2019; 96: 813–815 [DOI] [PubMed] [Google Scholar]
- 7. Urakawa I, Yamazaki Y, Shimada T et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006; 444: 770–774 [DOI] [PubMed] [Google Scholar]
- 8. Kuro-o M, Matsumura Y, Aizawa H et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997; 390: 45–51 [DOI] [PubMed] [Google Scholar]
- 9. Kurosu H, Yamamoto M, Clark JD et al. Suppression of aging in mice by the hormone Klotho. Science 2005; 309: 1829–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cambray S, Bermudez-Lopez M, Bozic M et al Association of a SNP combination pattern of Klotho gene with non-cardiovascular death in patients with chronic kidney disease. Clin Kidney J 2020; 10.1093/ckj/sfaa014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu MC, Shi M, Zhang J et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 2010; 24: 3438–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim K, Groen A, Molostvov G et al. α-Klotho expression in human tissues. J Clin Endocrinol Metab 2015; 100: E1308–E1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Borst MH, Vervloet MG, ter Wee PM et al Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol 2011; 22: 1603–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fernandez-Fernandez B, Izquierdo MC, Valiño-Rivas L et al. Albumin downregulates Klotho in tubular cells. Nephrol Dial Transplant 2018; 33: 1712–1722 [DOI] [PubMed] [Google Scholar]
- 15. Kuro-o M. The Klotho proteins in health and disease. Nat Rev Nephrol 2019; 15: 27–44 [DOI] [PubMed] [Google Scholar]
- 16. Semba RD, Cappola AR, Sun K et al. Plasma Klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci 2011; 66A: 794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pavik I, Jaeger P, Ebner L et al. Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: a sequence suggested from a cross-sectional study. Nephrol Dial Transplant 2013; 28: 352–359 [DOI] [PubMed] [Google Scholar]
- 18. Komaba H, Goto S, Fujii H et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int 2010; 77: 232–238 [DOI] [PubMed] [Google Scholar]
- 19. Andrukhova O, Slavic S, Smorodchenko A et al. FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO Mol Med 2014; 33: 229–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cha SK, Hu MC, Kurosu H et al. Regulation of renal outer medullary potassium channel and renal K excretion by Klotho. Mol Pharmacol 2009; 76: 38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brownstein CA, Adler F, Nelson-Williams C et al. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci USA 2008; 105: 3455–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu MC, Shi M, Zhang J et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 2011; 22: 124–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen TH, Kuro-O M, Chen CH et al. The secreted Klotho protein restores phosphate retention and suppresses accelerated aging in Klotho mutant mice. Eur J Pharmacol 2013; 698: 67–73 [DOI] [PubMed] [Google Scholar]
- 24. Smith RC, O’Bryan LM, Farrow EG et al. Circulating α Klotho influences phosphate handling by controlling FGF23 production. J Clin Invest 2012; 122: 4710–4715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karalliedde J, Maltese G, Hill B et al. Effect of renin-angiotensin system blockade on soluble Klotho in patients with type 2 diabetes, systolic hypertension, and albuminuria. Clin J Am Soc Nephrol 2013; 8: 1899–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takenaka T, Inoue T, Miyazaki T et al. Klotho ameliorates medullary fibrosis and pressure natriuresis in hypertensive rat kidneys. Hypertension 2018; 72: 1151–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Doi S, Zou Y, Togao O et al. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 2011; 286: 8655–8665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ding J, Tang Q, Luo B et al. Klotho inhibits angiotensin II-induced cardiac hypertrophy, fibrosis, and T dysfunction in mice through suppression of transforming growth factor-β1 signaling pathway. Eur J Pharmacol 2019; 859: 172549. [DOI] [PubMed] [Google Scholar]
- 29. Xia W, Zhang A, Jia Z et al. Klotho contributes to pravastatin effect on suppressing IL-6 production in endothelial cells. Mediators Inflamm 2016; 2016: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang X, Yalcin S, Lee DF et al. FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nat Cell Biol 2011; 13: 1092–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamamoto M, Clark JD, Pastor JV et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 2005; 280: 38029–38034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oh HJ, Nam BY, Lee MJ et al. Decreased circulating klotho levels in patients undergoing dialysis and relationship to oxidative stress and inflammation. Perit Dial Int 2015; 35: 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moreno JA, Izquierdo MC, Sanchez-Niño MD et al. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Neprhol 2011; 22: 1315–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou X, Chen K, Lei H et al. Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol 2015; 26: 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arking DE, Krebsova A, Macek M Sr et al. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA 2002; 99: 856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arking DE, Becker DM, Yanek LR et al. Klotho allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet 2003; 72: 1154–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arking DE, Atzmon G, Arking A et al. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res 2005; 96: 412–418 [DOI] [PubMed] [Google Scholar]
- 38. Citterio L, Delli Carpini S, Lupoli S et al. Klotho gene in human salt-sensitive hypertension. Clin J Am Soc Nephrol 2020; 15: 375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bigazzi R, Zagato L, Lanzani C et al. Hypertension in high school students: genetic and environmental factors: the HYGEF study. Hypertension 2020; 75: 71–78 [DOI] [PubMed] [Google Scholar]
- 40. Friedman DJ, Afkarian M, Tamez H et al. Klotho variants and chronic hemodialysis mortality. J Bone Miner Res 2009; 24: 1847–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhai C, Tang G, Qian G et al. Polymorphism of klotho G-395A and susceptibility of coronary artery disease in East-Asia population: a meta-analysis. Int J Clin Exp Med 2015; 8: 1582–1588 [PMC free article] [PubMed] [Google Scholar]
- 42. Memmos E, Sarafidis P, Pateinakis P et al. Soluble Klotho is associated with mortality and cardiovascular events in hemodialysis. BMC Nephrol 2019; 20: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xie J, Yoon J, An SW et al. Soluble Klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol 2015; 26: 1150–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saito Y, Nakamura T, Ohyama Y et al. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun 2000; 276: 767–772 [DOI] [PubMed] [Google Scholar]
- 45. Haruna Y, Kashihara N, Satoh M et al. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci USA 2007; 104: 2331–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Campos-Obando N, Lahousse L, Brusselle G et al. Serum phosphate levels are related to all-cause, cardiovascular and COPD mortality in men. Eur J Epidemiol 2018; 33: 859–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ravikumar P, Li L, Ye J et al. αKlotho deficiency in acute kidney injury contributes to lung damage. J Appl Physiol 2016; 120: 723–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hu MC, Shi M, Gillings N et al. Recombinant α-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int 2017; 91: 1104–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao M-M, Xu M-J, Cai Y et al. Mitochondrial reactive oxygen species promote p65 nuclear translocation mediating high-phosphate-induced vascular calcification in vitro and in vivo. Kidney Int 2011; 79: 1071–1079 [DOI] [PubMed] [Google Scholar]
- 50. Oh YS, Appel LJ, Galis ZS et al. National Heart, Lung, and Blood Institute Working Group report on salt in human health and sickness: building on the current scientific evidence. Hypertension 2016; 68: 281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Katsuya T, Rakugi H, Ogihara T. Inflammation and salt sensitivity in the early state of hypertension. Hypertens Res 2007; 30: 105–107 [DOI] [PubMed] [Google Scholar]
- 52. Kirabo A. A new paradigm of sodium regulation in inflammation and hypertension. Am J Physiol Regul Integr Comp Physiol 2017; 313: R706–R710 [DOI] [PMC free article] [PubMed] [Google Scholar]