Fig 2. Schematic depiction of insulin signaling during Diabetes and ATM deficiency.
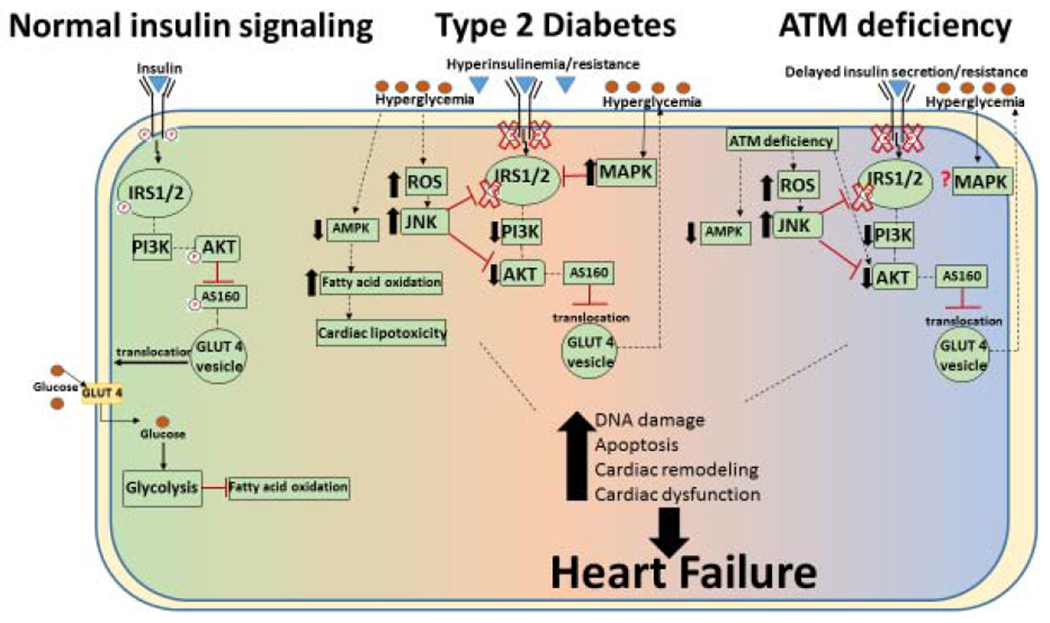
Left: Insulin responsive signaling: The intracellular signaling cascade begins with the binding of insulin to the insulin receptor which activates IRS1/2 (insulin receptor substrate proteins 1 and 2) and downstream targets such as Akt. Active Akt may phosphorylate Akt substrate 160 (AS160), and stimulate GLUT4 translocation to the plasma membrane and allowing glucose uptake. Center: Type 2 diabetic insulin signaling: Overproduction/release of insulin causes hyperinsulinemia. As insulin resistance increases, decreased insulin-mediated glucose uptake occurs resulting in decreased activation of IRS1/2 and Akt, phosphorylation of AS160, GLUT 4 translocation and glucose uptake. Persistent hyperglycemia then causes an increase in reactive oxygen species (ROS), and may activate JNK and decrease AMPK activation. These changes may shift the metabolism toward fatty acid oxidation and cardiac lipotoxicity. Together, these alterations can cause DNA damage, apoptosis, cardiac remodeling and cardiac dysfunction, leading to heart failure. Right: Insulin resistance during ATM deficiency: Delayed insulin secretion and insulin resistance is observed during ATM deficiency with decreased activation of IRS1/2 and Akt. This may decrease AS160 phosphorylation and glucose uptake. ATM deficiency independently associates with mechanisms suggested to cause insulin resistance such as decreased AMPK activation, increased JNK activation and higher oxidative stress. ATM deficiency also associates with increased DNA damage, apoptosis, cardiac remodeling and cardiac dysfunction.
