Abstract
High circulating trimethylamine-N-oxide (TMAO) is associated with an increased risk of cardiovascular disease and mortality in people with chronic kidney disease (CKD). In individuals with CKD, reduced kidney function leads to decreased excretion of TMAO, which results in accumulation in the circulation. Higher circulating TMAO has been linked to higher intake of animal-based foods in omnivorous diets. Thus, plant-based diets have been suggested as an intervention to slow the progression of CKD and reduce cardiovascular risk, perhaps explained in part by reduced TMAO production. This article reviews the current evidence on plant-based diets as a dietary intervention to decrease gut-derived TMAO production in patients with CKD, while highlighting methodological issues that present challenges to advancing research and subsequent translation of this approach. Overall, we find that plant-based diets are promising for reducing gut-derived TMAO production in patients with CKD but that further interventional studies are warranted.
Introduction
Cardiovascular disease (CVD) is the leading cause of death among patients with chronic kidney disease (CKD), and those who progress to kidney failure, or end-stage kidney disease (ESKD), have the greatest risk of mortality because of CVD.1 Recently, nontraditional risk factors such as the disturbance of the gut microbiota and, consequently, increased production of toxic metabolic byproducts, known as uremic retention solutes (URSs), have been linked to increased cardiovascular risk.2 Gut-derived trimethylamine-N-oxide (TMAO) is a topic of recent interest because increased serum levels of TMAO are associated with greater all-cause mortality,3,4 CVD,1,2,5,6 inflammation,7–10 and progression of CKD.5,11
Kidney function decline also occurs in tandem with a distinct, unfavorable shift in the composition of gut microbiota, including a lower relative abundance of potentially beneficial bacteria (including families Prevotellaceae and Lactobacillaceae) and a higher relative abundance of potentially harmful bacteria (e.g., from the family Enterobacteriaceae12).13–15 Diet and the pathophysiology of CKD may influence the composition and metabolism of the gut microbiota.14,16,17 Dietary interventions that limit the production of TMAO may be a mechanism to limit kidney disease progression and CVD in this population. Circulating levels of TMAO reflect increased intake of dietary precursors.18,19 Studies on both rodents and humans demonstrate that reducing intake of dietary precursors of TMAO may lower blood TMAO levels.18–21 Increased serum TMAO levels in the general population are associated with CVD.6 However, increased levels of TMAO are more concerning in CKD because the excretion of TMAO from circulation is dependent on kidney function. One study showed that circulating TMAO levels may be removed effectively by hemodialysis treatment.22 However, these therapies are only indicated in ESKD, making the reduction of serum TMAO levels in earlier stages of CKD a potential therapy of interest. This review provides an overview of the current literature focusing on plant-based diets as an intervention to target the microbial production of TMAO in CKD and provides perspectives on methodological issues that add further complexity to this area of study.
Trimethylamine and Trimethylamine N-Oxide Production
TMAO is a small, water-soluble molecule derived from multiple nutrients (e.g., choline, carnitine, betaine, phosphatidylcholine) found mostly in animal-based foods that is formed when trimethylamine (TMA) undergoes oxidation by the liver23 and, in the presence of molecular oxygen, some members of the gut microbiota.24 Foods containing choline, lecithin, and L-carnitine are dietary precursors endogenously modified to form TMA and ultimately TMAO. Choline is a substrate for bacteria that associate with glycyl radical enzymes, known as choline TMA-lyase, which works with glycyl radical enzymes activase to cleave TMA.25 In addition, L-carnitine and other derivatives, specifically gamma-butyrobetaine, serve as substrates for bacteria which use the 2-part Rieske-type carnitine oxygenase to form TMA.20,26 L-carnitine undergoes microbial modification to form gamma-butyrobetaine before cleavage of the TMA moiety.20 Most of the metabolic intermediates are absorbed via passive diffusion27 and travel to the liver for further modification. TMA can be oxidized to form TMAO through bacterial flavin-containing monooxygenases that are associated with intestinal mucosa or absorbed into the portal circulation and oxidized in the liver via hepatic flavin monooxygenase-3.28 However, the relative contribution of bacterial versus hepatic monooxygenase to the systemic pool of TMAO is unknown. TMAO is then released into the circulation and is excreted by the kidneys into the urine through tubular secretion.27,29 The relationship between dietary precursor intake and TMAO formation highlights a possible role for nutrition intervention to control TMAO levels in at-risk populations.
TMAO levels are significantly elevated in patients with CKD when compared with healthy controls.5,11,13,22,30–32 In the absence of kidney dysfunction, TMAO is cleared by the kidneys through tubular secretion, explaining the higher levels when kidney function is compromised to some extent. In addition, in marine organisms, TMAO serves a function as a protein-stabilizing molecule that appears to counteract the protein-destabilizing effects of high urea.33 This may be a physiologic reason for higher TMAO production in patients with CKD in response to higher levels of urea in the intestinal lumen.
Measuring TMAO in Healthy Individualsand CKD
Investigators setting out to quantify TMAO, both circulating and excreted, face several methodological challenges that can yield poorly comparable results. Control of variables such as metabolomic approach, measurement of dietary intake, and level of kidney function often vary greatly, and this is in addition to the large interindividual and intraindividual variation of circulating TMAO.34 Previous studies have used various analyses for the detection and quantification of fecal, urine, and serum metabolites, including nuclear magnetic resonance (NMR) spectroscopy and either liquid or gas chromatography followed by mass spectrometry (LC-MS or GC-MS, respectively).
Urine
There is strong evidence that a higher intake of specific sources of animal protein lead to increased excretion of TMAO in the urine across different metabolomic platforms. Miller et al.35 focused specifically on the intake of eggs in healthy adults (N = 6), which are high in phosphatidylcholine, and the impact they had on urine and serum levels of TMAO in a longitudinal, double-blinded, randomized controlled dietary intervention. Quantification by LC-MS showed a dose-dependent response of TMAO in the urine and serum correlated with increased egg dosage. Xu et al.36 found decreased urinary TMAO levels in lactovegetarians compared with omnivores by NMR. These findings agree with studies using NMR demonstrating that increased TMAO levels in the urine are related to increased animal protein intake.37–40 An experimental study in mice supplemented with dietary choline used multiple metabolomic approaches to measure TMAO in the serum including multinuclear NMR, LC-MS, and GC-MS.41 The results confirmed previous findings, suggesting that not all platforms are needed to capture the profile of the urine metabolite profiles.
Few studies have evaluated urinary TMAO levels in patients with CKD and ESKD and none have controlled dietary intake. Stubbs et al.30 measured urinary TMAO:creatinine ratio (urine TMAO μg: creatinine mg) and fractional excretion (%) with LC-MS in healthy controls (N = 12) and patients with CKD (N = 56, CKD stages 3–5 and not dialysis-dependent). Interestingly, there was no difference between urinary concentration or fractional excretion between healthy controls and patients with CKD. When these parameters were stratified by CKD stage (3a, 3b, 4–5), there was still no apparent difference in median values when compared with healthy controls, suggesting that TMAO excretion does not rise to accommodate increased production.
Serum
Multiple approaches concur that TMAO serum levels are elevated in individuals with the consumption of omnivorous diets compared with consumption of plant-based diets and individuals with CKD. Koeth et al.42 administered broad-spectrum antibiotics to participants in a feeding study with multiple high L-carnitine intake challenges and measured TMAO in the serum by LC-MS. After a week of antibiotic administration, the amount of endogenous TMAO in the plasma was suppressed after another L-carnitine challenge. In a longitudinal study, Tang et al.11 found increased serum TMAO levels in patients with CKD (estimated glomerular filtration rate, <60 mL/min per 1.73 m2) by LC-MS. Studies by Kaysen et al.31 and Bain et al.22 both found that serum TMAO levels were markedly increased in patients with CKD undergoing HD. However, Kaysen et al.31 noted that TMAO levels in their study were almost a magnitude lower than previously described in the study by Bain et al.22 arising from LC-MS measurement instead of GC-MS, with several other studies reporting similarly lower levels in healthy adults.6,11,42 Another study using GC-MS by Ozasa et al.43 found higher circulating TMAO levels in a healthy population than studies using LC-MS, which may also have been due to differences in ethnicity or methods. Together, these findings indicate that GC-MS may overestimate circulating TMAO levels.
Overall, these studies indicate a negative correlation between the circulating concentrations of TMAO and kidney function. However, the cause of this may be increased production, reduced tubular secretion, or both. Given the differences in recent findings, methodological approaches should be considered when quantifying serum TMAO levels as these differences may cause incomparable results.
Feces
Fecal metabolomics remain largely unexplored in the context of diet and CKD. Poesen et al.44 found that fecal metabolite profiles were altered in CKD compared with healthy controls in both humans and rats by GC-MS, though it should be noted that TMAO was not among the metabolites examined. Their novel study design included three control groups compared with individuals undergoing HD: healthy controls, age-matched healthy controls, and household contacts living with patients undergoing HD. However, the dietary intake of household contact controls was not assessed for similarity to patients undergoing HD. Despite the lack of dietary control and assessment, the household contacts and patients undergoing HD were similar compared with the other control groups simply from being exposed to the same home environment, which may include eating the same diet at home. These findings suggest that diet and environmental exposures affect the composition of the gut microbiota to a greater extent than CKD, though this was not directly assessed in this study. A review by Karu et al.45 noted that many studies using more than one analysis platform provided greater metabolite coverage in the fecal metabolome.45
The Gut Microbial Community is Altered in CKD
The composition of the microbiota has been described as unfavorably disturbed in patients with CKD compared with healthy populations.14 The relationship between CKD and the gut microbiome is most likely bidirectional: pathophysiological or dietary changes that occur with CKD progression may affect the gut microbiota, and disturbed gut microbiota present in CKD may also contribute to further CKD progression and increased risk for cardiovascular events and overall mortality. Aranov et al.46 found that several URS were either completely missing or significantly reduced in the serum from patients with CKD without a colon, suggesting that some URS originate in the colon.
Studies Evaluating the Composition of the Gut Microbiota in CKD
Currently, there are few studies evaluating the composition of the gut microbiota in patients with CKD, particularly in nondialysis populations. Of the existing literature in this area, methodological approaches varied in these studies (some of which are now outdated) and the reporting of outcomes make synthesis of these studies challenging. Table 1 describes studies of the gut microbiota in various stages of CKD in humans and animal models, the molecular methods used, and results on the composition of the gut microbiota in CKD. Vaziri et al.14 were the first to describe a difference in community composition, directly measuring microbial DNA in the gut microbiomes of patients with ESKD and rats via microarrays. Relative abundances of members of the phyla Actinobacteria (especially genera Brachybacterium and Nesterenkonia), Firmicutes (within class Clostridia, especially from families Catabacteraceae and Peptostreptococcaceae and genus Catenibacterium within the class Mollicutes), and Proteobacteria (especially members of Gammaproteobacteria, specifically the families Enterobacteriaceae, Halomonadaceae, Methylococcaceae, Moraxellaceae, and Pseudomonadaceae as well as the genera Alteromonas and Thiothrix) were significantly increased in patients undergoing HD compared with healthy controls. To control for interindividual genetic variability, feces from rats with CKD (5/6 nephrectomy) displayed similar increases in abundance as found in the humans. This was one of the first studies to clearly reveal that gut microbiota in CKD display lower bacterial diversity than the gut microbiota in healthy individuals. One drawback to microarrays is that measurement is limited to just those taxa represented by oligonucleotide probes on the chip, which may not reflect the total microbial community structure well. Accordingly, many investigators have moved away from using microarray owing to this limitation and moved toward high-throughput sequencing of the 16S rRNA gene.
Table 1.
Studies Evaluating the Composition of the Gut Microbiota in Human and Animal Models of CKD
| Author/Yr | Population | Microbiota in CKD | Molecular Method | Conclusion |
|---|---|---|---|---|
| Nishiyama 201947 | Mice 5/6 Nx sham, (N = 4) 5/6 Nx, (N = 4) |
Increased genera:
Allobaculum, Bifidobacterium, and Turicibacter in 5/6 Nx mice. Decreased genera: Lactobacillus, Oscillospira, and unclassified Ruminococcaceae significantly decreased in the 5/6 Nx group. Unclassified Rikenellaceae was also decreased (not significant) in the 5/6 Nx mice group. |
16S, V3-V4 region | 5/6 Nx altered gastrointestinal motility and caused constipation by altering the gut microbiota |
| Lun 201917 | Healthy, n = 24 CKD, n = 49 13/49 HD Mean eGFR (n/a) |
Increased genera: Bacteroides, Escherichia_Shigella, Parabacteroides, Ruminococcus, Weissella, Flavonifractor, Ruminiclostridium, Sellimonas, Erysipelatoclostridium, Eggerthella, and Clostridium. More abundant in HC: Dialister, Eubacterium, Carnobacterium, Lachnospira, Subdoligranulum, Eubacterium_coprostanoligenes, Coprococcus2, Roseburia, Ruminococcaceae, Romboutsia, Butyricicoccus, Collinsella, Eubacterium, Tyzzerella. |
16S, V3-V4 region | The composition of gut microbiota was different in CKD populations compared with healthy populations, and Lachnospira and R._gnavus were identified as microbial biomarkers. |
| Al-Obaide 201732 | Healthy, n = 20 CKD-T2DM, n = 20 Mean eGFR (16.54 ± 3.01 mL/min/1.72 m2) |
Increased genera:
Anaerococcus, Desulfitobacter, Enterococcus, Streptococcus, Desulfovibrio, Klebsiella, Pseudomonas, Citrobacter
(TMA producers:
Clostridium, Escherichia, Enterobacter, Acinetobacter, Proteus and Lactobacillus). Decreased genera: Bifidobacterium |
16S, V3-V4 region | Changes in the gut microbiota were associated with increased levels of TMA and TMAO, and increased gut permeability in patients with T2DM-CKD |
| Liu 201848 | Rats 5/6 Nx sham, N = 8 5/6 Nx, N = 10 |
Markedly altered genera in second and tenth week: Chlamydiales, Rhodospirillales, Rhodobacterales, Rhizobiales, Xanthomonadales, Lactobacillales, Legionellales, Rhodocyclales | 16S, region (n/a) | The gut amino acid metabolism profile was disordered with CKD progression, which was highly related to the gut microbiota dysbiosis and metagenome change. |
| Xu 201713 | Healthy, N = 32 CKD stage 4 (15–30 mL/min/per 1.73 m2), N = 16 CKD stage 5 (<15 mL/min/per 1.73 m2), N = 16 |
Increased genera:
Enterococcus and Clostridium in patients with CKD. Decreased genera: Prevotella, Coprococcus, Megamonas, Sutterella, Enterobacter, Acidaminococcus, Dorea, and Roseburia were more abundant in healthy controls. |
16S, V4 region | Patients with CKD showed different dominant bacteria and altered genes expression, which involved in choline, betaine, L-carnitine and trimethylamine metabolism, compared to the control group. |
| Vaziri 201314 | Healthy, N = 12 ESKD, N = 24 Mean eGFR (n/a) RATS 5/6 Nx sham, N = 5 5/6 Nx, N = 6 |
Increased genera
from Brachybacterium, Catenibacterium, Enterobacteriaceae, Halomonadaceae, Moraxellaceae, Nesterenkonia, Polyangiaceae, Pseudomonadaceae, and Thiothrix families in patients undergoing HD when compared with the control group. Decreased prevalence of Bacteroidetes and Firmicutes in the 5/6 Nx rats, especially Lactobacillaceae and Prevotellaceae, when compared with the sham group. |
16S, Microarray chip | Uremia alters the composition of the gut microbiota in ESKD |
| Black 201849 | Nondialysis CKD stages 3–4, N = 30 (N = 14 adhered to diet) Mean eGFR of adhesion group (36.5 ± 11.7 mL/min/per 1.73 m2) |
Genera not identified | PCR-DGGE of 16S gene | Change in gut microbiota profiles observed, but the samples were too variable to form clusters in analysis. The average number of bands was positively associated with protein intake (r = 0.44, P = .04) |
| Barros 2015 | Healthy, N = 19 Stage 3–4 CKD, N = 20 Mean eGFR (n/a) |
Presence of Listeria monocytogenes and Flavobacteriaceae bacterium in patients with CKD. Lachnospiraceae bacterium and Butyrivibrio crossotus in healthy individuals. |
PCR-DGGE of 16S gene | CKD patients did not present altered gut microbial profile, however sequencing of bands suggests different microbiota between groups. |
CKD, chronic kidney disease; DGGE, denaturing gradient gel electrophoresis; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; HD, hemodialysis; PCR, polymerase chain reaction; TMA, trimethylamine; TMAO, trimethylamine-N-oxide
The 16S rRNA is a highly conserved gene in bacteria that encodes for the 16S ribosomal RNA. By sequencing one or several regions of this gene, relative abundances of bacterial groups present in the gut microbiota can be assessed, and differences depending on diet, disease, and other factors can be revealed.50 Al-Obaide et al.32 used 16S rRNA sequencing to confirm that the genera Clostridium, Escherichia, Enterobacter, Acinetobacter, Proteus, and Lactobacillus relative abundances were higher and Bifidobacterium was lower in patients with diabetic nephropathy. Lun et al.17 confirmed the altered composition of the gut microbiome in CKD and sought microbial biomarkers of CKD progression. Results showed a higher abundance of 13 taxa, including Bacteroides, Escherichia/Shigella, Parabacteroides, Ruminococcus gnavus, Ruminococcus torques, Weissella, Flavonifractor, Ruminiclostridium5, Sellimonas, Erysipelatoclostridium, Eggerthella, and Clostridium innocuum. A study by Xu et al.13 examined both the gut microbiota and serum TMAO levels in patients with CKD stages 4–5 (N = 32) and healthy controls (N = 32). They found a higher relative abundance of the genera Enterococcus and Clostridium and lower relative abundances of members of the genera Prevotella, Coprococcus, Megamonas, Sutterella, Enterobacter, Acidaminococcus, Dorea, and Roseburia in patients with CKD. Experimental models of 5/6 nephrectomized mice and rats by Nishiyama et al.47 and Liu et al.48 confirmed the unfavorable shifts associated with CKD progression found in humans. It is important to note that Liu et al.48 measured the changes in the gut microbiota of CKD as it progressed in rats and found that the gut microbiota became increasingly different over time. Taken together, these studies address the differences in the composition of the gut microbiota of patients with CKD and healthy people.
Changes in the Gut Microbial Community Affect TMAO Production
Alterations in microbiota composition have the potential to lead to changes in the TMA/TMAO pathway. Wang et al.41 found that when the microbiota of C57BL/6J Apoe−/− mice were suppressed after the administration of broad-spectrum antibiotics, the circulating levels of TMAO were significantly reduced compared with mice with preserved microbiota (no antibiotics). In relation to TMA cleavage, Rath et al.51 used quantitative polymerase chain reaction and Illumina sequencing to quantify and characterize the TMA-producing potential in fecal samples from healthy adults (N = 154). They found that bacterial genes coding for choline TMA-lyases were present in Clostridium cluster XIVa and Eubacterium genomes, and L-carnitine oxygenases were identified from the class Gammaproteobacteria, primarily from the Escherichia coli species. Comprehensive findings from Xu et al.13 strengthened the current evidence regarding the contribution of disturbed gut microbiota in CKD to higher circulating TMAO levels. Two groups of mice received fecal microbiota transplants from either patients with CKD or healthy controls. They found relatively higher amounts of bacterial genes associated with TMAO reductase, phosphatidylcholine synthase, lipopolysaccharide cholinephosphotransferase, and choline monooxygenase, which were associated with higher luminal levels of TMAO of the mice transplanted with fecal microbiota from patients with CKD when compared with mice with samples from healthy controls. Higher levels of these TMAO-related bacterial genes may have been altered in patients with CKD for biochemical alterations because of reduced kidney function or differences in dietary restrictions, though it should be noted that dietary intake was not measured/collected in this study.
Dietary intervention studies show that diet plays a role in shaping the composition and function of the gut microbiome in CKD and may be a useful therapeutic target to slow CKD progression.52–54 However, all human studies comparing the CKD population to healthy adults lack a controlled-feeding component, which could significantly impact results as diet is one of the greatest confounding factors of the gut microbiota and CKD management includes dietary restrictions that may be inconsistently adopted across patients.
Dietary Components Alter the Gut Microbial Community in CKD
Few studies have evaluated effects of dietary interventions on the microbiome in either humans or animal models of CKD. In a longitudinal study by Black et al.,49 patients with nondialysis CKD (N = 14) followed a low-protein diet (0.6 g/kg/day) for 6 months. This study compared the denaturing gradient gel electrophoresis profiles of 16S rRNA gene amplicons. Using this technique, a change in the gut microbiota profile was observed after the dietary intervention. However, it was not possible to separate the samples in clusters owing to the low similarity between the generated profiles. This is likely due to the individuality of the gut microbiome, small sample size adhering to the diet for the full 6-month duration, and/or the relatively low sensitivity of the denaturing gradient gel electrophoresis method compared with amplicon sequencing approaches. Therefore, there is a need for controlled feeding studies to evaluate how the response of the gut microbiota in patients with CKD compares with the response in healthy populations.
Dietary Methods in CKD to Change the Gut Microbiome to Alter TMAO or to Directly Change TMAO
Plant-based protein intake has been shown to decrease blood pressure and delay CKD progression.55,56 Plant-based diets include an increased intake of whole grains, fruits, vegetables, nuts, and legumes with various levels of animal protein omitted: “vegetarians” consume no animal protein, “lactovegetarians” consume dairy, “lacto-ovo vegetarians” consume dairy and eggs, and “vegans” consume no animal-based protein or byproducts.57 Omnivorous diets are high in animal protein (meat, eggs, fish, dairy) when compared with plant-based diets of vegans and vegetarians and typically lead to higher production of TMAO. However, not all sources of protein lead to increased TMAO production.58
Recent studies in healthy adults indicate that the chronic consumption of L-carnitine, found primarily in red meat, leads to greater systemic increases of TMAO levels than other animal protein products.18,19 In a recent study by Koeth et al.,18 healthy omnivores and vegetarians/vegans ingested isotopically labeled TMAO precursors after oral antibiotics, or following chronic (≥2 months) L-carnitine supplementation. Those following an omnivorous dietary pattern were found to have higher systemic TMAO owing to increased microbial TMA/TMAO production from carnitine, but not choline, and reduced renal TMAO excretion. On discontinuing the intake of dietary red meat for 4 weeks, plasma TMAO levels were significantly reduced. Wang et al.19 studied the effect of chronic dietary intake of red meat, white meat, and nonmeat protein on TMAO metabolism and excretion in healthy individuals in a randomized three-period crossover design. Participants (N = 113) consumed a baseline diet for 2 weeks, followed in random order by three experimental diets with higher intake of (1) red meat, (2) white meat, and (3) nonmeat protein. Results showed that only chronic dietary intake of red meat was associated with significantly higher blood and urine TMAO levels, reduced fractional TMAO excretion rate, and higher TMA/TMAO production from L-carnitine, but not choline. TMAO levels were significantly decreased after discontinuing intake of red meat for 4 weeks. Currently, there are no controlled feeding studies with a crossover design evaluating the effects of dietary pattern on TMA/TMAO production, metabolism, and excretion in patients with CKD.
Long-term dietary intake influences the structure and function of the gut microbiota.59–62 However, David et al.16 demonstrated that even short-term dietary interventions of plant-based or animal-based diets rapidly alter the fecal microbiota and the metabolites produced in healthy individuals. In studies comparing omnivorous diets with plant-based diets, circulating levels of TMAO are significantly lower in plant-based diets.42 Plant-based diets may reduce circulating levels of TMAO because of synergistic interactions with other dietary components. The combination of a lower intake of TMA/TMAO precursors and a higher intake of dietary fiber that promotes commensal microbial growth, may reduce serum TMAO. However, these claims currently have not been assessed in experimental or clinical studies of CKD.
A group of anaerobic archaea, known as methanogens, may be key in reducing TMA available for absorption in the distal colon. Methanogenic archaea are capable of harvesting methylated amines from TMA, resulting in reduced levels of TMA available for absorption and oxidation. Ramezani et al.63 colonized C57BL/6 mice fed a high choline/TMA-supplemented diet with five methanogenic species and found that all species were able to colonize in the gut and significantly lower circulating levels of TMAO. The relative abundance of these species can vary greatly in humans, potentially accounting for the high interindividual variability in circulating TMAO levels seen in most studies. While no controlled feeding studies with patients with CKD have investigated methanogenic archaea species, a study by Nava et al.64 showed that healthy individuals consuming a dietary pattern low in animal products had a higher diversity of methanogenic archaea species than the group that reported consuming higher amounts of animal products.
Recently, plant-based diets have been suggested as an intervention in earlier stages of CKD to help slow its progression of CKD by decreasing urinary phosphorus excretion.65,66 As previously described, a reduction in dietary TMA/TMAO precursors and increased dietary fiber may make plant-based diets a useful intervention to reduce serum levels of TMAO by modulating the composition and function of the gut microbiota. Studies are needed to differentiate how dietary patterns and dietary precursors of the TMA/TMAO pathway may alter the composition of the gut microbiome and TMAO production in CKD.
Metabolomic Measurements of TMAO and the Gut Microbiome in CKD and Methodological Considerations
Analyte Detection and Identification Method Selection
Advancements in technology and methods have improved the measurement and characterization of fecal, urine, and serum metabolites. Studying the kinetics and quantification of metabolites is important for developing clinical biomarkers, which can be used to detect, diagnose, and treat diseases including CKD. One study combined epidemiological and metabolomic approaches and found that elevated plasma TMAO levels predicted the development of CKD even after adjustment for estimated glomerular filtration rate, age, sex, diabetes, hypertension, and proteinuria at baseline.67 The platform used to analyze metabolites depends on the research question. For targeted analysis, with the goal of quantifying TMAO, LC-MS or NMR have provided similar results. For an untargeted (unbiased) analysis, which measures broad swaths of metabolites, GC-MS is a good choice for identifying TMAO in the context of the metabolome. Targeted analyses solely seeking to quantify TMAO may be better suited with methods that provide increased sensitivity for lower abundance polar molecules, such as LC-MS or NMR.68 In addition, for studies aiming to quantify relatively low-abundance metabolites, internal standards should be used to account for variation in samples.69 Further information on the analysis of serum, urine, and fecal metabolites can be found in the studies by Bouatra et al.,70 Karu et al.,45 Moraes et al.,71 and Psychogios et al.68
Controlling for Confounding Variables in CKD when Studying the Gut Microbiome
Disease Effects
CKD disproportionately affects ethnic minorities older than 45 years of age, and therefore, there may be age- and ethnicity-specific differences along with interindividual differences that inject noise and reduce the ability to detect differences in microbiota composition.72–74 Investigators should consider stratifying data by ethnicity owing to the impact that ethnicity-related changes may exert on the composition of the gut microbiota.75 Brooks et al.76 also highlights a need to account for ethnic variation as a confounding factor when studying the relationship of gut microbiota to disease. Their study evaluated the relationship between self-declared ethnicity and gut microbiota differences in two large data sets: the American Gut Project (N = 1,375) and the Human Gut Microbiome Project (N = 298).77,78 Findings indicate that genetic patterns of ancestral ethnic variation may play a role in shaping the gut microbiota. Stratification by race and ethnicity may be useful to determine the true effect of the intervention when studying the gut microbiome in human populations. Another potential confounder in assessing microbiome is antibiotics. There is variability in the recovery time of antibiotics, and previous studies that suggest patients treated with antibiotics within the last 6 months should be excluded,79 although the feasibility of this approach may be difficult. Medications commonly prescribed in CKD have been shown to influence the microbiome, including iron-based and non–calcium-based phosphate binders.80 Non–calcium-based phosphate binders, such as sevelamer, are known to bind some gut-derived microbial metabolites in patients with ESKD.81 The use of proton pump inhibitors (PPIs) is also a commonly prescribed medication in CKD populations. Seto et al.82 found that prolonged use of PPIs in healthy adults leads to reduced microbial diversity in the gut; however, this microbial disturbance was reversed with the cessation of PPI use after 1 month.
Diet Effects
Long-term dietary intervention studies are costly, and investigators often struggle with the recruitment and adherence of participants. It is important to consider the acceptance and palatability of the dietary intervention to increase adherence. Our study showed that individuals with CKD found a 70% plant-based protein diet palatable, resulting in good compliance for the duration of the study.65 Dietary restrictions are less commonly prescribed for patients with early-to-moderate stage CKD. However, individuals undergoing HD often require dietary restrictions for fluid, potassium, phosphorus, and sodium intake, which also need to be taken into consideration when designing and evaluating dietary interventions targeting the microbiome.
Conclusions and Future Research Needs
The gut microbiome is a promising target for therapeutic interventions in CKD. Current evidence suggests that modifying protein source and/or fiber intake to modulate the gut microbiota in individuals with CKD may be a useful approach to lower TMAO levels. Future dietary intervention studies in CKD should be tightly controlled owing to the large number of variables influencing the gut microbiome, including medications and race/ethnicity among other factors. Investigations of mainly plant-based diets that allow for limited amounts of animal protein may be most promising for broad implementation for patients accustomed to a typical Western omnivorous diet with a goal of reducing TMAO production.
Uncited Figure
Figure 1.
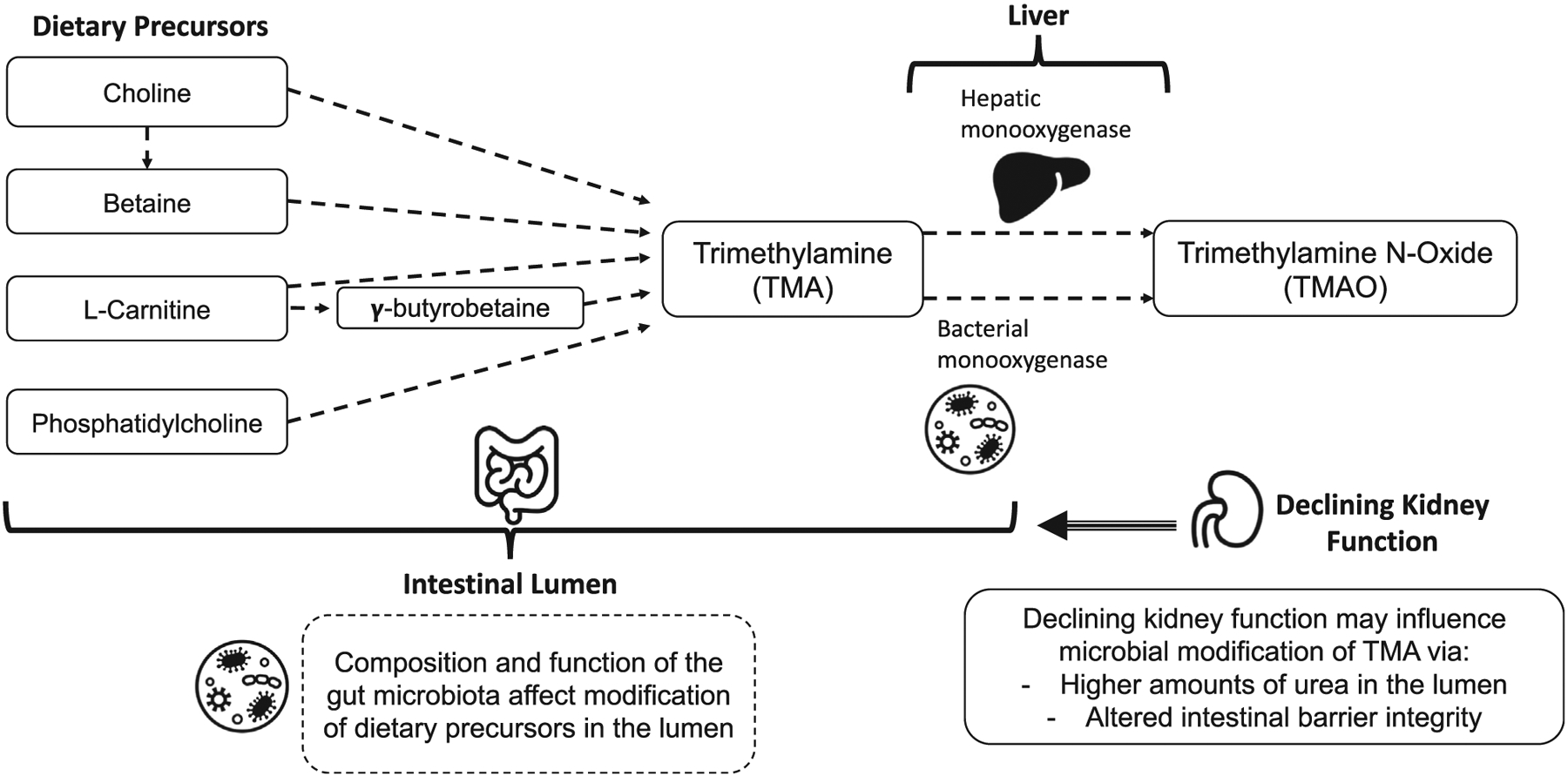
The relationship between dietary intake of TMA/TMAO precursors and TMAO production. The amount of dietary precursors ingested, the composition and function of the gut microbiota, and monooxygenase function contribute to the production of TMA/TMAO. Dietary precursors (choline, betaine, L-carnitine, and phosphatidylcholine) are ingested and modified in the lumen of the gastrointestinal tract, largely in the colon, by members of the gut microbiota to form trimethylamine. This modification can vary based on the composition of microbial members that are present and their function based on the biochemical state of the lumen (pH). Some gut bacteria possess the flavin-containing monooxygenase (FMO) as well, resulting in some oxidation of TMA in the lumen to form trimethylamine N-oxide (TMAO). The remaining TMA is absorbed across the apical intestinal membrane where it is taken up into hepatic circulation. Upon arrival in the liver, TMA can also be oxidized by hepatic flavin monooxygenase 3 (FMO3) to form TMAO. As kidney function declines, urea levels rise in the intestinal lumen, resulting in altered epithelial barrier integrity. Through these changes to the microenvironment of the intestinal lumen, the composition of the gut microbiota may also lend to changes seen in TMAO-forming activity.
Support:
This work was partially supported by NIH grants K01 DK102864 (KMHG), K23 DK102824 (RNM), T32 AR065971 (AB), R01 DK11087103 and UL1 TR002529 (SMM) and Veterans Affairs grant I01 BX001471 (SMM).
Footnotes
Financial Disclosure: A.B. reports honoraria from Amgen.
References
- 1.Kim RB, Morse BL, Djurdjev O, et al. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016;89:1144–1152. [DOI] [PubMed] [Google Scholar]
- 2.Qi J, You T, Li J, et al. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med. 2018;22:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gruppen EG, Garcia E, Connelly MA, et al. TMAO is associated with mortality: impact of Modestly impaired renal function. Sci Rep. 2017;7:13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng Y, Li Y, Rimm EB, et al. Dietary phosphatidylcholine and risk of all-cause and cardiovascular-specific mortality among US women and men. Am J Clin Nutr. 2016;104:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Missailidis C, Hällqvist J, Qureshi AR, et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One. 2016;11:e0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen ML, Zhu XH, Ran L, Lang HD, Yi L, Mi MT. Trimethylamine-N-Oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J Am Heart Assoc. 2017;6:e006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T, Chen Y, Gua C, Li X. Elevated circulating trimethylamine N-oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Front Physiol. 2017;8:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohrmann S, Linseisen J, Allenspach M, von Eckardstein A, Müller D. Plasma concentrations of trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a German adult population. J Nutr. 2016;146:283–289. [DOI] [PubMed] [Google Scholar]
- 10.Andersen K, Kesper MS, Marschner JA, et al. Intestinal dysbiosis, barrier dysfunction, and bacterial translocation account for CKD-related systemic inflammation. J Am Soc Nephrol. 2017;28:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu KY, Xia GH, Lu JQ, et al. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci Rep. 2017;7:1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaziri ND, Wong J, Pahl M, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–315. [DOI] [PubMed] [Google Scholar]
- 15.Vaziri ND, Zhao YY, Pahl MV. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transpl. 2016;31:737–746. [DOI] [PubMed] [Google Scholar]
- 16.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lun H, Yang W, Zhao S, et al. Altered gut microbiota and microbial biomarkers associated with chronic kidney disease. Microbiologyopen. 2019;8:e00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koeth RA, Lam-Galvez BR, Kirsop J, et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J Clin Invest. 2019;129:373–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Bergeron N, Levison BS, et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koeth RA, Levison BS, Culley MK, et al. γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Roberts AB, Buffa JA, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bain MA, Faull R, Fornasini G, Milne RW, Evans AM. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transpl. 2006;21: 1300–1304. [DOI] [PubMed] [Google Scholar]
- 23.Janeiro MH, Ram ırez MJ, Milagro FI, Mart ınez JA, Solas M. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients. 2018;10:1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Patel NA, Crombie A, Scrivens JH, Murrell JC. Bacterial flavin-containing monooxygenase is trimethylamine monooxygenase. Proc Natl Acad Sci U S A. 2011;108:17791–17796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A. 2012;109:21307–21312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Y, Jameson E, Crosatti M, et al. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci U S A. 2014;111:4268–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teft WA, Morse BL, Leake BF, et al. Identification and characterization of trimethylamine-N-oxide uptake and efflux transporters. Mol Pharm. 2017;14:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shih DM, Wang Z, Lee R, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu W, Bush KT, Nigam SK. Key role for the organic anion transporters, OAT1 and OAT3, in the in vivo handling of uremic toxins and solutes. Sci Rep. 2017;7:4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stubbs JR, House JA, Ocque AJ, et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. 2016;27:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaysen GA, Johansen KL, Chertow GM, et al. Associations of trimethylamine N-oxide with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J Ren Nutr. 2015;25:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Obaide MAI, Singh R, Datta P, et al. Gut microbiota-dependent trimethylamine-N-oxide and serum biomarkers in patients with T2DM and advanced CKD. J Clin Med. 2017;6:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yancey PH. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol. 2005;208(Pt 15):2819–2830. [DOI] [PubMed] [Google Scholar]
- 34.Kühn T, Rohrmann S, Sookthai D, et al. Intra-individual variation of plasma trimethylamine-N-oxide (TMAO), betaine and choline over 1 year. Clin Chem Lab Med. 2017;55:261–268. [DOI] [PubMed] [Google Scholar]
- 35.Miller CA, Corbin KD, da Costa KA, et al. Effect of egg ingestion on trimethylamine-N-oxide production in humans: a randomized, controlled, dose-response study. Am J Clin Nutr. 2014;100:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J, Yang S, Cai S, Dong J, Li X, Chen Z. Identification of biochemical changes in lactovegetarian urine using 1H NMR spectroscopy and pattern recognition. Anal Bioanal Chem. 2010;396:1451–1463. [DOI] [PubMed] [Google Scholar]
- 37.Lenz EM, Bright J, Wilson ID, et al. Metabonomics, dietary influences and cultural differences: a 1H NMR-based study of urine samples obtained from healthy British and Swedish subjects. J Pharm Biomed Anal. 2004;36:841–849. [DOI] [PubMed] [Google Scholar]
- 38.Stella C, Beckwith-Hall B, Cloarec O, et al. Susceptibility of human metabolic phenotypes to dietary modulation. J Proteome Res. 2006;5:2780–2788. [DOI] [PubMed] [Google Scholar]
- 39.Dumas ME, Maibaum EC, Teague C, et al. Assessment of analytical reproducibility of 1H NMR spectroscopy based metabonomics for large-scale epidemiological research: the INTERMAP Study. Anal Chem. 2006;78:2199–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasmussen LG, Winning H, Savorani F, et al. Assessment of the effect of high or low protein diet on the human urine metabolome as measured by NMR. Nutrients. 2012;4:112–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozasa H, Shimizu M, Koizumi A, Wakabayashi A, Yamazaki H. Trimethylamine generation in patients receiving hemodialysis treated with l-carnitine. Clin Kidney J. 2014;7:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poesen R, Windey K, Neven E, et al. The influence of CKD on colonic microbial metabolism. J Am Soc Nephrol. 2016;27:1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karu N, Deng L, Slae M, et al. A review on human fecal metabolomics: methods, applications and the human fecal metabolome database. Anal Chim Acta. 2018;1030:1–24. [DOI] [PubMed] [Google Scholar]
- 46.Aronov PA, Luo FJ, Plummer NS, et al. Colonic contribution to uremic solutes. J Am Soc Nephrol. 2011;22:1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishiyama K, Aono K, Fujimoto Y, et al. Chronic kidney disease after 5/6 nephrectomy disturbs the intestinal microbiota and alters intestinal motility. J Cell Physiol. 2019;234:6667–6678. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Li J, Yu J, et al. Disorder of gut amino acids metabolism during CKD progression is related with gut microbiota dysbiosis and metagenome change. J Pharm Biomed Anal. 2018;149:425–435. [DOI] [PubMed] [Google Scholar]
- 49.Black AP, Anjos JS, Cardozo L, et al. Does low-protein diet influence the uremic toxin serum levels from the gut microbiota in nondialysis chronic kidney disease patients? J Ren Nutr. 2018;28:208–214. [DOI] [PubMed] [Google Scholar]
- 50.Hugerth L, Anders A. Analyzing microbial community composition through amplicon sequencing: from sampling to hypothesis testing. Front Microbiol. 2017;8:1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rath S, Heidrich B, Pieper DH, Vital M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome. 2017;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwashita Y, Ohya M, Yashiro M, et al. Dietary changes involving bifidobacterium longum and other nutrients delays chronic kidney disease progression. Am J Nephrol. 2018;47:325–332. [DOI] [PubMed] [Google Scholar]
- 53.Kieffer DA, Piccolo BD, Vaziri ND, et al. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am J Physiol Ren Physiol. 2016;310:F857–F871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaziri ND, Liu SM, Lau WL, et al. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One. 2014;9:e114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azadbakht L, Shakerhosseini R, Atabak S, Jamshidian M, Mehrabi Y, Esmaill-Zadeh A. Beneficiary effect of dietary soy protein on lowering plasma levels of lipid and improving kidney function in type II diabetes with nephropathy. Eur J Clin Nutr. 2003;57:1292–1294. [DOI] [PubMed] [Google Scholar]
- 56.Azadbakht L, Atabak S, Esmaillzadeh A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy: a longitudinal randomized clinical trial. Diabetes Care. 2008;31:648–654. [DOI] [PubMed] [Google Scholar]
- 57.Melina V, Craig W, Levin S. Position of the academy of nutrition and dietetics: vegetarian diets. J Acad Nutr Diet. 2016;116:1970–1980. [DOI] [PubMed] [Google Scholar]
- 58.Clarys P, Deriemaeker P, Huybrechts I, Hebbelinck M, Mullie P. Dietary pattern analysis: a comparison between matched vegetarian and omnivorous subjects. Nutr J. 2013;12:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muegge BD, Kuczynski J, Knights D, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332:970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Markiewicz LH, Honke J, Haros M, Świątecka D, Wróblewska B. Diet shapes the ability of human intestinal microbiota to degrade phytate–in vitro studies. J Appl Microbiol. 2013;115:247–259. [DOI] [PubMed] [Google Scholar]
- 62.Walker AW, Ince J, Duncan SH, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramezani A, Nolin TD, Barrows IR, et al. Gut colonization with methanogenic archaea lowers plasma trimethylamine N-oxide concentrations in apolipoprotein e−/− mice. Sci Rep. 2018;8:14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nava GM, Carbonero F, Ou J, Benefiel AC, O’Keefe SJ, Gaskins HR. Hydrogenotrophic microbiota distinguish native Africans from African and European Americans. Environ Microbiol Rep. 2012;4:307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moorthi RN, Armstrong CL, Janda K, Ponsler-Sipes K, Asplin JR, Moe SM. The effect of a diet containing 70% protein from plants on mineral metabolism and musculoskeletal health in chronic kidney disease. Am J Nephrol. 2014;40:582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clegg DJ, Hill Gallant KM. Plant-based diets in CKD. Clin J Am Soc Nephrol. 2019;14:141–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rhee EP, Clish CB, Ghorbani A, et al. A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol. 2013;24:1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Psychogios N, Hau DD, Peng J, et al. The human serum metabolome. PLoS One. 2011;6:e16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bouatra S, Aziat F, Mandal R, et al. The human urine metabolome. PLoS One. 2013;8:e73076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moraes C, Fouque D, Amaral AC, Mafra D. Trimethylamine N-oxide from gut microbiota in chronic kidney disease patients: focus on diet. J Ren Nutr. 2015;25:459–465. [DOI] [PubMed] [Google Scholar]
- 72.Peralta CA, Bibbins-Domingo K, Vittinghoff E, et al. APOL1 genotype and race differences in incident albuminuria and renal function decline. J Am Soc Nephrol. 2016;27:887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gebregziabher M, Ward RC, Taber DJ, et al. Ethnic and geographic variations in multimorbidty: evidence from three large cohorts. Soc Sci Med. 2018;211:198–206. [DOI] [PubMed] [Google Scholar]
- 74.Stubbs JR, Stedman MR, Liu S, et al. Trimethylamine N-oxide and cardiovascular outcomes in patients with end-stage kidney disease receiving maintenance hemodialysis. Clin J Am Soc Nephrol. 2019;14:261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. [DOI] [PubMed] [Google Scholar]
- 76.Brooks AW, Priya S, Blekhman R, Bordenstein SR. Gut microbiota diversity across ethnicities in the United States. Plos Biol. 2018;16:e2006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McDonald D, Birmingham A, Knight R. Context and the human microbiome. Microbiome. 2015;3:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Consortium HMP. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4554–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Biruete A, Hill Gallant KM, Lindemann SR, Wiese GN, Chen NX, Moe SM. Phosphate binders and nonphosphate effects in the gastrointestinal tract. J Ren Nutr. 2020;30:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goto S, Yoshiya K, Kita T, Fujii H, Fukagawa M. Uremic toxins and oral adsorbents. Ther Apher Dial. 2011;15:132–134. [DOI] [PubMed] [Google Scholar]
- 82.Seto CT, Jeraldo P, Orenstein R, Chia N, DiBaise JK. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42. [DOI] [PMC free article] [PubMed] [Google Scholar]


