Fig. 2.
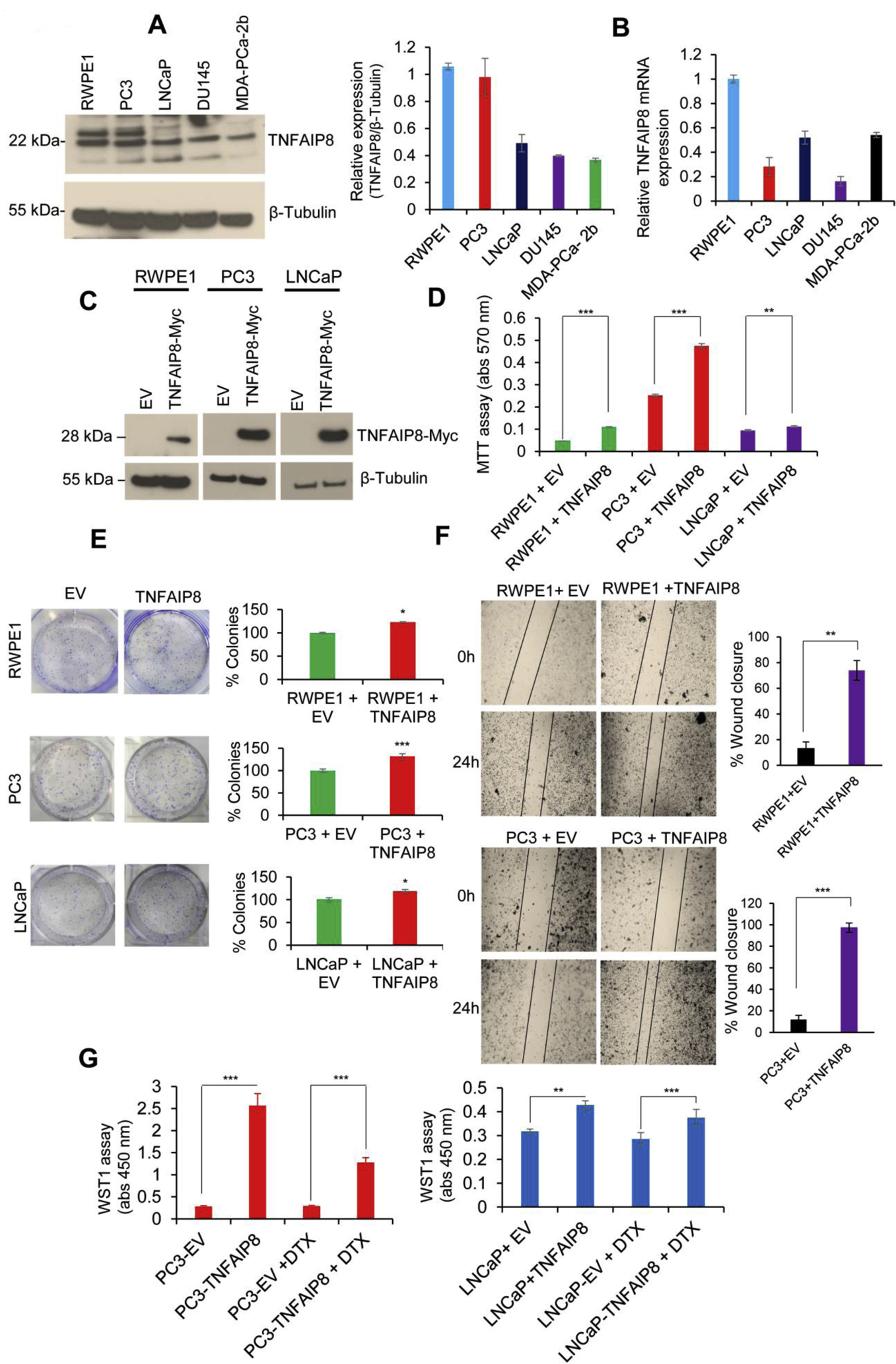
TNFAIP8 enhances cell metabolic activity, cell viability, and colony formation in PCa cells. (A) RWPE1, PC3, LNCaP, DU145, and MDA-PCa-2b cells (1×105) were grown in six-well plates for 40h, and cell lysates (50 μg) were immunoblotted with TNFAIP8 and β-tubulin antibodies. TNFAIP8 protein levels were quantified using ImageJ software (https://imagej.nih.gov/ij/) and plotted (left panel). (B) Normal and PCa cells (1×105) were grown in six-well plates for 72h, and expression of relative TNFAIP8 mRNA levels was analyzed by RT/qPCR as described in the materials and methods section. (C) RWPE1, PC3, LNCaP cells were transfected with EV or TNFAIP8-Myc tagged plasmid (2 μg) for 30h, and expression of TNFAIP8-Myc tagged protein in RWPE1 and PCa cells were analyzed by western blotting. (D) Similarly, EV and TNFAIP8-Myc transfected cells (1×104) were re-plated in 96 well plates, and the effect of TNFAIP8 expression on cell survival was analyzed by MTT assay. (E) RWPE1, PC3, LNCaP cell were transfected with EV or TNFAIP8-Myc plasmids and transfected cells (3 × 103) were re-plated in six-well plates for 7 to 10 days and effect of TNFAIP8 expression on cell colony formation were measured and plotted (left and right panels). (F) Wound healing assay: RWPE1 and PC3 cells were transfected with EV or TNFAIP8-Myc plasmid for 30 h. The effect of the TNFAIP8-Myc expression on cell migration was analyzed by wound-healing assay. Representative images of the wound healing assay (left panels) and the calculated scratch area are shown (right panels). (G) PC3 and LNCaP cells were transfected with EV or TNFAIP8-Myc plasmid for 24h and treated with DTX (1nM) for an additional 30h, and cell proliferation was analyzed by WST1 assay. Data represent mean ± SEM from three independent experiments. *P<0.05, **P<0.01, ***P<0.001 compared to EV transfected cells.
