Abstract
Introduction
Peroxisome proliferator–activated receptor (PPAR) subfamily play an important role in chondrogenesis. Previous study has reported that mixture of GW0742 (PPAR-δ agonist), hyaluronic acid (HA) and mesenchymal stem cells (MSCs) enhance chondrogenesis. The purpose of this study is to compare with efficacies of commercially available HA and demonstrate correlation of PPAR-γ and PPAR-δ.
Methods
In this experimental study, MSCs were cultured with chondrogenic media and clinical HA gels (Euflexxa®, Synvisc®, Orthovisc® and Supartz®) using micormass culture method. Expression of type Ⅰ, Ⅱ collagen and matrix metalloprotease-13 (MMP-13) was measured by immunoblotting. MSCs were cultured with chondrogenic media and/or HA and/or GW0742 and/or rosiglitazone (PPAR-γ agonist) and/or human osteoarthritis synovial fluid. Immunoblotting was used to measure expression of type Ⅱ collagen and PPAR-γ. To identify the effective dose for chondrogenesis and adipogenesis, either 0.1, 1, 5 or 10 μM of rosiglitazone was added to MSCs in chondrogenic media or adipogenic media.
Results
Clinical HA gels inhibited expression of type Ⅰ collagen and enhanced the expression of MMP-13. Type Ⅱ collagen expression was significantly elevated in all treatment groups except Supartz®. GW0742 decreased the expression of PPAR-γ with/without inflammation condition. Rosiglitazone enhanced adipogenesis in a dose-dependent manner and enhanced the expression of type Ⅱ collagen under inflammation condition. Otherwise, the expression of type Ⅱ collagen and formation of chondrocyte spheroids showed a dose-dependent manner with a peak at 1 μM of rosiglitazone.
Conclusions
PPAR-γ has a considerable anti-inflammatory effect and a strong pro-adipogenic effect, which inhibits the chondrogenic effect. PPAR-γ is related with PPAR-δ and shows a chondrogenic effect at lower concentrations. And clinical HA gels shows various efficacy of chondrogenesis. This study suggested that PPAR-γ and PPAR-δ are key regulatory factors of chondrogenesis.
Keywords: Mesenchymal stem cells, PPAR-γ, PPAR-δ, Chondrogenesis, Adipogenesis, Type Ⅱ collagen
Abbreviations: α-MEM, α-minimum essential medium; DJD, degenerative joint disease; ECM, extracellular matrix; FBS, fetal bovine serum; GAG, glycosaminoglycans; HA, hyaluronic acid; MMP, matrix metalloprotease; MSC, mesenchymal stem cells; OA, osteoarthritis; PBS, phosphate-buffered saline; PPAR, Peroxisome proliferator–activated receptor; TGF, Transforming growth factor
Graphical abstract
1. Introduction
Osteoarthritis (OA) is a progressive, inflammatory joint disorder characterized clinically by pain, stiffness, crepitus and in severe cases, complete destruction of the joint cartilage. Since cartilage has a limited ability to self-regenerate, once damaged or in the process of aging, it undergoes degradation. OA is a major contributor to disability in the United States, with an expected dramatic increase over the next 20 years due to an increasingly aging population [1].
Functional cartilage consists of type Ⅱ collagen-producing chondrocytes embedded within an extracellular matrix (ECM) composed of collagens (type IV, V, IX and XI) and proteoglycans comprised of protein core and glycosaminoglycans (GAG) [2]. One of the important components of articular cartilage is hyaluronic acid (HA). HA is a non-sulfated GAG that is widely distributed throughout connective tissues. Upon binding to aggrecans via link proteins, HA forms large negatively charged aggregates that imbibe water and mediate the compressive, shear, and tensile resistance of cartilage [3]. HA enhances proteoglycan synthesis in the equine model [4]. HA provides pores of optimum size that facilitates the infiltration and chondrogenesis of mesenchymal stem cells (MSCs) [5,6]. Therefore, HA is very important for the function of articular cartilage.
OA was once thought to be a degenerative joint disease (DJD) but is now regarded as an inflammatory form of arthritis where inflammatory mediators contribute to the deterioration of the cartilage matrix. In osteoarthritic joints, inflammatory cytokines, chemokines and inflammatory mediators increase the expression of matrix metalloproteases (MMPs) and aggrecanases in damaged chondrocytes, resulting in increased secretion of MMPs and aggrecanases that degrade cartilage ECM [[7], [8], [9], [10]]. HA has a strong anti-OA property demonstrated by suppressed oxidative stress and inflammation [11,12] and decreased MMP expression [13]. It is not surprising with the therapeutic properties and benefits of HA on chondrogenesis. HA by itself can ameliorate the symptoms of OA [14,15]. There are several well-known HA types available for clinical application. Although the varied clinical response to the different types of HA is known [16], to our knowledge, a comparative evaluation of the efficacies of different HA types in the formation of cartilage has not been done. More specifically, their efficacies for the differentiation of MSCs into chondrocyte, the key initial process of cartilage regeneration, have yet to be described.
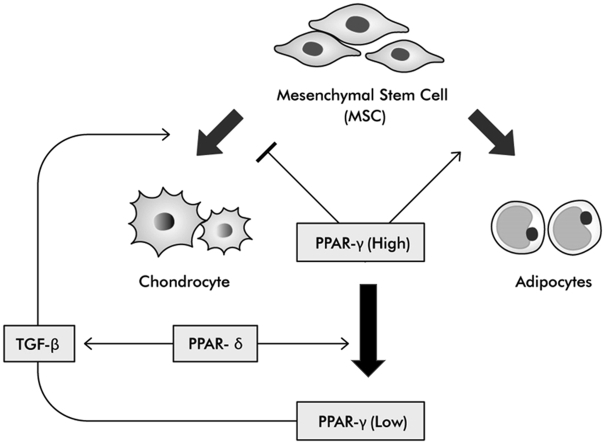
Peroxisome proliferator–activated receptors (PPARs) are ligand-activated transcription factors and a member of the nuclear hormone receptor superfamily. PPARs have three isoforms (PPAR-α, δ/β, γ) and play important roles in fat and glucose homeostasis [17]. PPAR-δ are almost ubiquitously expressed, and the function of PPAR-δ is related to fat metabolism and apoptosis [18]. PPAR-γ are highly expressed in adipocytes but are also found in a range of tissues. PPAR-γ play an important role in inflammation, fat metabolism, glucose homeostasis and adipogenesis [19]. Recently, various authors reported that PPAR-δ and PPAR-γ are related to chondrogenesis. Three isoforms of PPAR receptors were expressed in growth plate chondrocytes [20]. PPAR-γ knockout mice exhibited abnormal cartilage growth and development [21] and showed accelerated spontaneous osteoarthritis phenotype [22]. However, the function of the PPAR subfamily in chondrogenesis remains unclear. A previous study focused on PPAR-δ and reported that PPAR-δ selective agonist (GW0742) showed a strong chondrogenic effect and great synergic effect with HA [23]. Therefore, this study examined the chondrogenic effect of PPAR-γ in vitro. The anti-inflammatory effect [19] and pro-adipogenic effect of PPAR-γ have already been reported [24]. The chondrogenic effect of PPAR-δ and HA have also reported [23]. This study shows a comparison between these properties of PPAR-γ and PPAR-δ.
This study clearly demonstrates that there are different efficacies among commercially available HA types in the generation of Type Ⅱ collagen, a marker of functional hyaline cartilage. These data also demonstrated the chondrogenic effect and adipogenic effect of PPAR-γ with or without inflammation condition and its correlation with PPAR-δ.
2. Methods
2.1. Isolation of bone marrow derived MSCs
In this experimental study, frozen human bone marrow mononuclear cells were used for isolation (AllCells, Alameda, CA, USA) [25]. Cells were resuspended in α-minimum essential medium (α-MEM) supplemented with 20% fetal bovine serum (FBS) and 1% antibiotic-antimycotic solution (Invitrogen, Grand Island, NY, USA). Mononuclear cells were plated at a density of 1–5 × 106 cells per 75 cm2 flask and cultured at 37 °C in a 5% CO2 incubator. After 48 h, the medium over the MSCs were replaced with fresh medium every 3–4 days for 15 days. The initial density of plated cells was 1 × 106 cells/75 cm2 flask and cells were cultured until they reached 70–80% confluence.
2.2. Chondrogenesis with HA micromass culture
Chondrogenic differentiation was performed by three-dimensional and high-density micromass culturing. MSCs were subcultured 2–3 times and plated in a 75 cm2 flask, followed by culturing in α-MEM with 20% FBS for 1 day at 37 °C with and initial density of 1.5 × 106 cells/cm2. On the following day, MSCs were harvested from the 75 cm2 flask, and 80,000 cells were re-plated at a density of 1.6 × 107 cells/ml in preloaded 70 μL HA gels (Euflexxa®, 1% sodium hyaluronate; Ferring Pharmaceuticals Inc., Parsippany, NJ, USA) on a 96-well plate or 0.1 mg/mL HA on a 24-well tissue culture plate and incubated in chondrogenesis medium (STEMPRO® Chondrogenesis Differentiation Kit, Gibco-Life Technology, Grand Island, NY, USA) for 2 h at 37 °C in a 5% CO2 incubator. Clinical HA gels including Euflexxa® (Ferring Pharmaceuticals Inc., Parsippany, NJ, USA), Synvisc® (Genzyme Biosurgery, Ridgefield, NJ, USA), Orthovisc® (Anika Therapeutics, Inc., Bedford, MA, USA), or Supartz® (Seikagaku Co., Tokyo, Japan) were used for three-dimensional (3-D) culture. After 2 h, the medium over the cells mass was replaced with fresh chondrogenesis medium every 2–3 days and incubated for 15 days. A control group of human bone marrow (hBM) derived MSCs was incubated without chondrogenic medium.
2.3. Chondrogenesis with PPAR-γ/-δ agonist
A group of hBM-MSCs (0.3 106 cells) were incubated in chondrogenic medium with GW0742 for 14 days, while a control group was incubated without chondrogenic medium. In order to compare the inflammation situation, human osteoarthritis (OA) synovial fluid was used. In order to compare the correlation of PPAR-γ and type Ⅱ collagen under inflammation conditions, another group of hBM-MSCs was incubated in chondrogenic medium with/without 1 mM GW0742 and with/without HA (70 mL Euflexxa®) in 50% human OA synovial fluid for 14 days. Euflexxa® was used as a representative merchandized hyaluronic acid because of chondrogenic ability and cost-effectiveness [23]. The mixture of GW0742, HA and MSCs is named by Chondrogenic Hyaluronic Acid–Mesenchymal Stem Cells–PPAR-δ agonist (CHAMP). On the 14th day, chondrocytes were processed for immunoblotting using antibodies to type Ⅱ collagen, PPAR-γ and β-actin. The expression of type Ⅱ collagen, PPAR-γ and β-actin was measured to examine the efficacy of chondrogenesis and the correlation between PPAR-δ and PPAR-γ.
In order to compare chondrogenic ability of PPAR-γ and PPAR-δ agonist under inflammation conditions, various combinations were used for experiments. hBM-MSCs (0.3 106 cells) was incubated in chondrogenic medium with 50% human OA synovial fluid (control) for 14 days. Either 1 mM GW0742 or 1 μM rosiglitazone (Sigma-Aldrich) was added in this mixture to compare chondrogenic capacity of PPAR-γ and PPAR-δ agonist. Three groups (control, rosiglitazone, GW0742) with/without 70 mL Euflexxa® were used to identify and compare synergic effect with/without HA. The expression of type Ⅱ collagen and β-actin was measured by immunoblotting.
The expression of type Ⅱ collagen and transforming growth factor-beta (TGF-β) and formation of chondrocyte spheroids were measured to identify the effective dose of rosiglitazone for chondrogenesis. hBM-MSCs were incubated with rosiglitazone (0.1, 1, 5 or 10 μM), HA (70 mL Euflexxa®) and chondrogenic medium for 14 days. The expression of type Ⅱ collagen, TGF-β and β-actin were measured by immunoblotting. To measure formation of chondrocyte spheroid formation, either 0.1, 1, 5 or 10 μM rosiglitazone was added to hBM-MSC with HA (70 mL Euflexxa®) and chondrogenic medium. Quantification of chondrocyte spheroids was performed three times (on the 7th, 10th and 14th days).
2.4. Quantification of chondrocyte spheres
In order to compare the size and number of chondrocyte spheroids, Alcian blue was used. First, chondrocyte pellets in each well were washed with phosphate-buffered saline (PBS, Sigma-Aldrich, St. Louis, MO, USA). The washed pellets were fixed with 4% formaldehyde in PBS for 30 min and stained with 1% Alcian Blue in 0.1 N HCl (Sigma-Aldrich, St. Louis, MO, USA) for 10 min. Cell pellets were washed three times with 0.1 N HCl for pH neutralization and then washed with distilled water. The images of stained cell pellets were taken using a light microscope. The number and size of chondrocyte sphere were quantified using ‘Metamorph’ software (Molecular Devices, Inc., Downingtown, PA, USA).
2.5. Examination of the expression of proteins chondrocytes
Chondrocyte pellets were dissolved in citrate-EDTA buffer (55 mM/5 mM; pH 6.8) for 10 min at 37 °C and centrifuged at 10 K rpm for 15 s. Then, the cell pellet was washed five times with PBS and resuspended in 200 μL of SDS-NuPAGE buffer for NuPAGE gel (Invitrogen, Grand Island, NY, USA) running. Immunoblotting was performed using monoclonal antibodies against Type Ⅰ collagen, Type Ⅱ collagen (1:1000 dilution; EMD Millipore, Billerica, MA, USA), MMP-13 (Abcam, Cambridge, MA, USA) TGF-β (1:1000 mg/mL; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and PPAR-γ (Abcam, Cambridge, MA, USA). The loading control, β-actin, will be detected using anti-β-actin antibody (1:10,000 dilution; Abcam, Cambridge, MA, USA). Secondary antibodies tagged horseradish peroxidase (HRP) (rabbit for TGF-β 1:20,000 dilution and mouse for type Ⅱ collagen and β-actin, 1:5000 dilution) was used. Proteins separated in the gel and the gel were transferred to nitrocellulose membrane (GE Healthcare Life Sciences, Pittsburgh, PA, USA) using TE77XP semidry blotter with 10 V for 3 h (Hoefer, Inc., Holliston, MA, USA). Protein band signals on blots were detected on Amersham Hyperfilm which was enhanced chemiluminescence (GE Healthcare Life Sciences, Pittsburgh, PA, USA) using SuperSignal West Pico Chemiluminescent Substrate kit (Thermo Fisher Scientific, Rockford, IL, USA).
2.6. Adipogenesis
In order to identifying the effect of PPAR-γ on adipogenesis and the effective dose of rosiglitazone for adipogenesis, either 0.1, 1, 5 or 10 μM rosiglitazone was added to hBM-MSC in adipogenic medium. MSCs (passages 2–3) were plated in a 75 cm2 flask at a density of 1.5 × 106 cells/cm2 and cultured in α-MEM with 20% FBS for 1 day at 37 °C. On the next day, MSCs were harvested from the 75 cm2 flask and 80,000 cells were re-plated at a density of 1.6 × 107 cells/ml on a 24-well tissue culture plate and incubated in adipogenic medium for 2 h at 37 °C in a 5% CO2 incubator. The adipogenic medium consisted of complete culture medium supplemented with DMEM-high glucose, 10% FBS, 10 μg/mL insulin, 0.5 mM dexamethasone (Sigma–Aldrich, St. Louis, MO), 0.5 mM isobutylmethylxanthine (Sigma–Aldrich, St. Louis, MO) and 0.1 mM indomethacin (Sigma–Aldrich, St. Louis, MO). In order to identify the effective dose of rosiglitazone for adipogenesis, 0.1, 1, 5 or 10 μM rosiglitazone was added to hBM-MSCs in adipogenic media.
On the 14th day, adipogenic differentiation medium was removed and the cells were washed with Dulbecco's modified phosphate-buffered saline (DPBS) without Ca++ and Mg++ (Thermo Fisher Scientific) and fixed for 30 min with a 4% formaldehyde solution (Sigma-Aldrich). DPBS washing was performed two times, and the cells were stained for 30 min with Oil Red O solution (Sigma-Aldrich). After the staining, DPBS washing was performed two more times. Adipocytes were quantified by optical density (OD), which was measured at wavelength of 405 nm.
2.7. Statistical analyses
The Fisher method of analysis of multiple comparisons (P < 0.05) was used to determine statistical significance between experimental group. For comparison between treatment groups, a single-factor analysis of variance (ANOVA) for multiple groups or the unpaired t-test for two groups was used. Differences between experimental groups were evaluated with ANOVA with Bonferroni corrections. Statistical significance was set at p < 0.05.
3. Results
3.1. Comparison of chondrogenesis efficacy between various HA gels
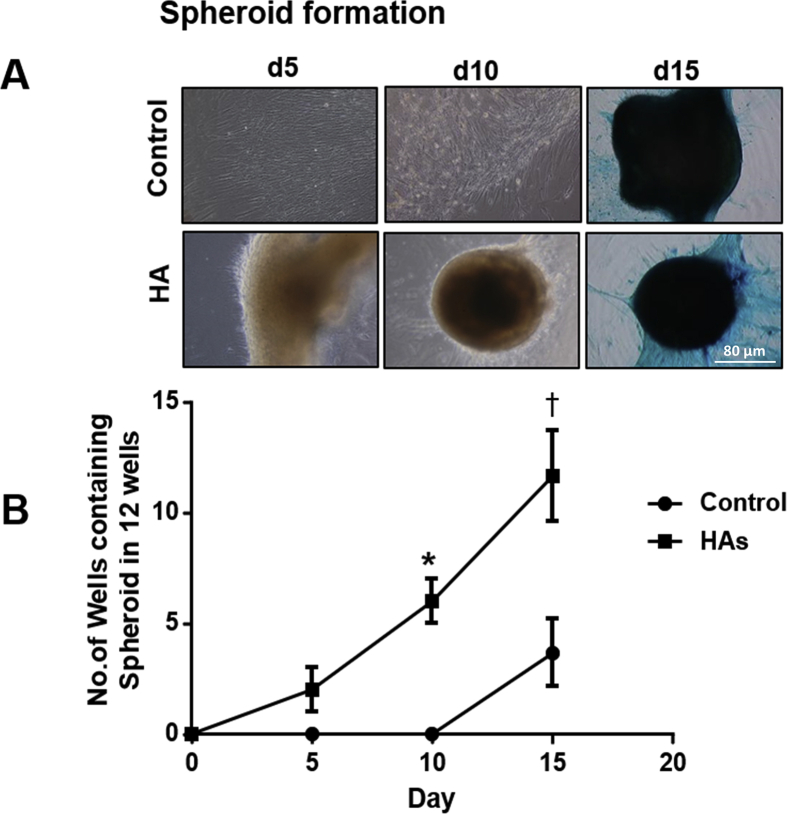
We measured the spheroid formation at day 5, 10 and 15 on both the control and the MSCs treated with 0.1 mg/mL HA mixed with chondrogenic media. This demonstrated increased formation of spheroid (Suppl. 1 A). On the 15th day, there was an increased number of spheroids formed compared to the control (Suppl. 1 B).
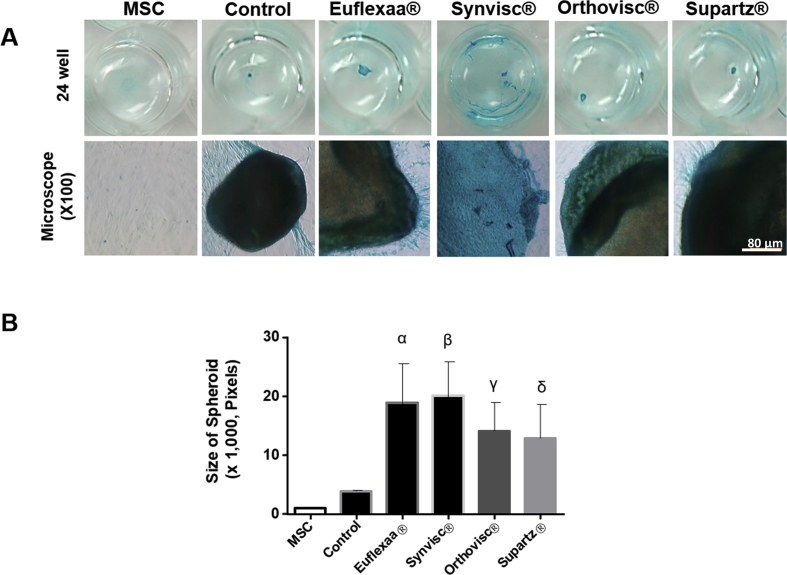
We examined whether or not cultivation of hMSCs on different types of hyaluronan gels enhanced chondrogenesis to different extents. hMSCs were seeded either on matrigel-coated wells (control) or on 60 μL of hyaluronan gel (Euflexxa®, Orthovisc®, Supartz®, or Synvisc®) in the wells of 96-well plates and incubated in chondrogenic medium for 15 days. The images of chondrocytes and chondrocyte spheroids on different types of hyaluronic acids were taken using a light microscope (Suppl. 2 A). On the 15th day of chondrogenesis, spheroids on Euflexxa® appeared to be more compacted and surrounded by denser GAGs compared to those on Orthovisc® and Supartz®. Compared to spheroids formed on HA gels, those formed on the culture plate without HA gel were shrunken and GAGs formation was reduced. Then, in order to quantify the extents of chondrogenesis, we measured the size of spheroids that were formed on the control and on the various HA gels after 15 days. Euflexxa® showed the highest efficacy in the formation of large chondrocyte spheroids compared to the other HA gels and the control. Synvisc® showed the second highest efficacy in the formation of large chondrocytes (Suppl. 2 B).
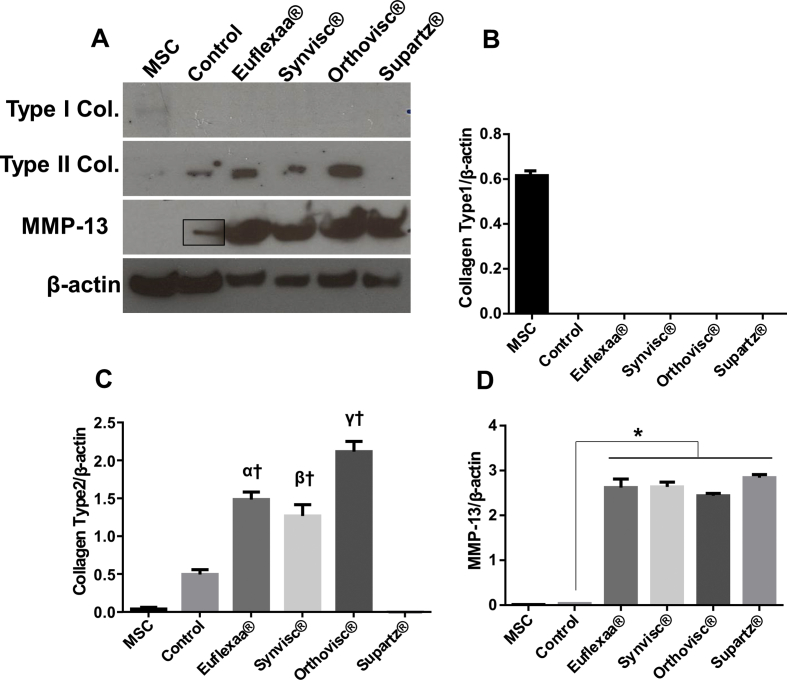
Protein expression analysis of untreated cultures and cultures treated with HA gel cultures were performed using Western blot analysis. Results show that Type Ⅰ collagen expression was only evident in MSCs (Fig. 1a and b). Type Ⅱ collagen expression was significantly elevated in all treatment groups except Supartz®, when compared to the control (Fig. 1a and c). Given that MMP-13 induces cartilage degradation, MMP-13 expression was highly elevated in all treatment groups (Fig. 1a and d).
Fig. 1.
Comparison of chondrogenesis efficacy between various HA gels. Chondrocytes differentiated 80,000 cells of hMSCs were seeded either on matrigel-coated wells on 60 μL of hyaluronan gel (Euflexxa®, Orthovisc®, Supartz® and Synvisc®) or without HA (control) in the wells of 96-well plates and incubated in chondrogenic medium for 15 days. (a) The expression of Type Ⅰ collagen, Type Ⅱ collagen and MMP-13 was measured by immunoblot analysis. (b) The expression of Type Ⅰ collagen was only shown in MSC group. (c) Type Ⅱ collagen expression was significantly elevated in all treatment groups except Supartz®. There was no statistical difference among Euflexxa®, Orthovisc® and Supartz®, (α, β, γ P > 0.05) (d) MMP-13 expression was highly elevated in all HA treatment groups.
3.2. Treatment with PPAR-δ agonist inhibits the expression of PPAR-γ
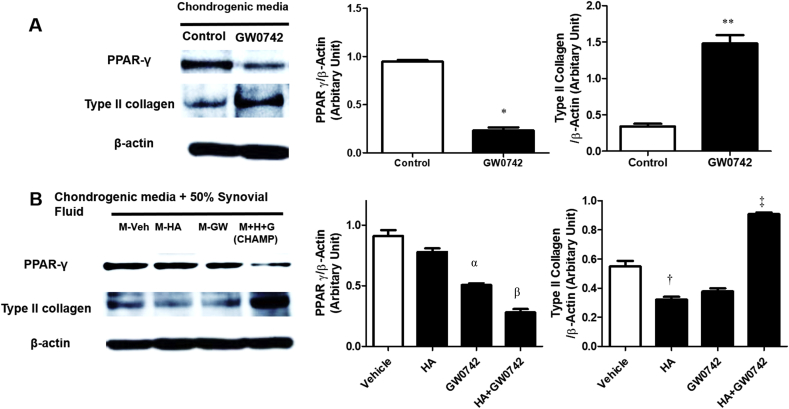
Given that the PPAR-δ agonist is a good chondrogenesis enhancer, we examined the correlation between PPAR-δ and PPAR-γ. PPAR-δ agonist (GW0742) was administered every second day for 14 days. Immunoblotting was performed using the antibodies to type Ⅱ collagen, PPAR-γ and β-actin (loading control). GW0742 increased the expression of type Ⅱ collagen, while inhibiting the expression of PPAR-γ (Fig. 2a).
Fig. 2.
Treatment with PPAR-δ agonist inhibits the expression of PPAR-γ. (a) MSCs were cultured with chondrogenic media and PPAR-δ agonist (GW0742) was administered every other day for 14 days. GW0742 increased the expression of type Ⅱ collagen, while inhibiting the expression of PPAR-γ. ∗P < 0.05 versus control. ∗∗P < 0.05 versus control (b) MSCs were cultured plus/minus 1 μM GW0742 to chondrogenic medium with 50% human OA synovial fluid for 14 days. The complete combination of hBM-MSCs, GW0742, and HA gel (CHAMP) significantly increased the protein expression of type Ⅱ collagen and inhibited the expression of PPAR-γ within human OA synovial fluid. αP < 0.05 versus control. βP < 0.05 versus control. †P < 0.001 versus control. ‡P < 0.05 versus control.
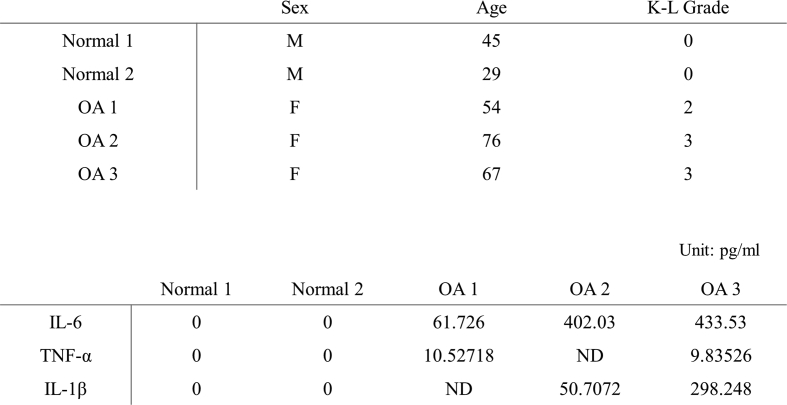
We examined whether the relationship between PPAR-δ and PPAR-γ was maintained under inflammation condition. We incubated hBM-MSCs (0.3 × 106 cells) on plus/minus 70 μL Euflexxa for 2 h. When cells were aggregated, we added plus/minus 1 μM GW0742 to chondrogenic medium with 50% human OA synovial fluid and incubated the combinations for 14 days. Addition of either GW0742 or HA gel to hBM-MSCs did not increase the expression of type Ⅱ collagen and significantly decreased the expression of PPAR-γ under inflammation condition. Human OA synovial fluid contains significantly higher concentration of pro-inflammatory cytokine such as IL-6, TNF-α, IL-1β than healthy synovial fluid (Suppl. 3). In contrast, the complete combination of hBM-MSCs, GW0742, and HA gel (CHAMP) significantly increased the protein expression of type Ⅱ collagen and inhibited the expression of PPAR-γ within human OA synovial fluid. When the expression of PPAR-γ decreases, the expression of type Ⅱ collagen shows an increasing trend under the PPAR-δ treatment condition (Fig. 2b).
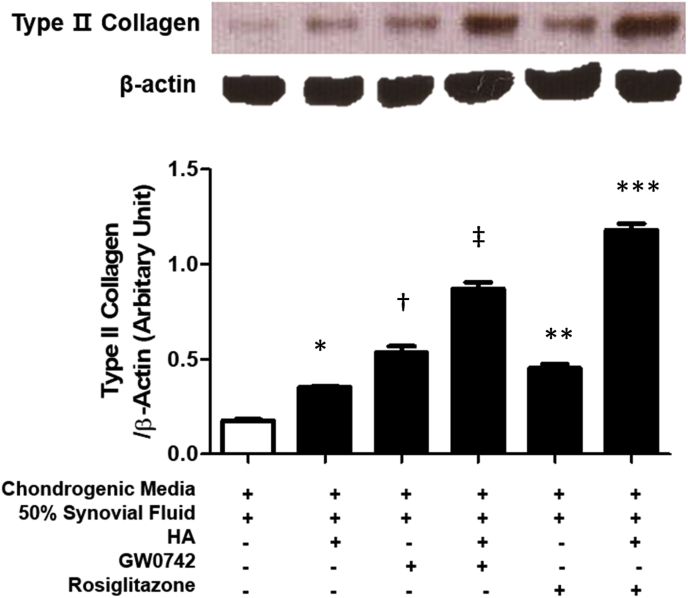
In order to compare the chondrogenic effect of PPAR-γ with that of the PPAR-δ agonist under inflammation conditions, we incubated hBM-MSCs (0.3 × 106 cells) on plus/minus 70 μL Euflexxa® for 2 h. When cells were aggregated, we added plus/minus 1 μM GW0742 or 1 μM rosiglitazone to chondrogenic medium with 50% human OA synovial fluid and incubated the combinations for 14 days. Then, we examined the extent of chondrogenesis by quantifying the expression levels of type Ⅱ collagen, given that the PPAR-δ agonist with HA and MSCs (CHAMP) is a strong inducer of chondrogenesis. Rosiglitazone with HA significantly enhanced the production of type Ⅱ collagen (Fig. 3). There are several studies that show that collagen induced arthritis was improved by rosiglitazone treatment in mice [26]. However, the type Ⅱ collagen expression capacity of rosiglitazone has not yet been reported. It is novel data which shows the synergism effect of HA and rosiglitazone and the chondrogenic effect of rosiglitazone.
Fig. 3.
Synergistic effect of PPAR-γagonist (rosiglitazone) and HA. MSCs were cultured plus/minus 70 μL Euflexxa® for 2 h, plus/minus 1 μM GW0742, plus/minus 1 μM rosiglitazone to chondrogenic medium with 50% human OA synovial fluid for 14 days. The expression of type Ⅱ collagen were significantly enhanced in treatment of PPAR-ã agonist (Rosiglitazone) and HA. ∗P < 0.001 versus control. †P < 0.05 versus control. ‡P < 0.05 versus control. ∗∗P < 0.05 versus control. ∗∗∗P < 0.05 versus control.
3.3. Treatment with a PPAR-γ agonist increases chondrogenesis at proper concertation
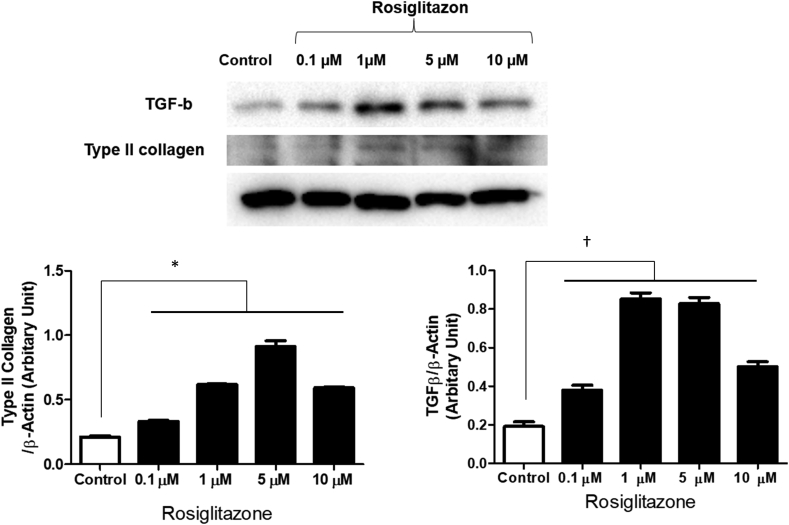
We examined whether a PPAR-γ agonist can function as a chondrogenic agent for MSCs. First, we examined the extent to which incubation of hBM-MSCs with rosiglitazone, a PPAR-γ agonist, increases TGF-β and type Ⅱ collagen production. We treated hBM-MSCs in HA gel (Euflexxa®) every second day with 0, 0.1, 1, 5, or 10 μM of rosiglitazone in chondrogenic media for 14 days. Then, chondrocytes were processed for immunoblotting using the antibodies to type Ⅱ collagen and TGF-β (β-actin: loading control). Rosiglitazone increased the protein level of TGF-β in a dose-dependent manner with a peak at 1 μM (Fig. 4). We then examined whether rosiglitazone enhances the expression of type Ⅱ collagen as it did for TGF-β. Rosiglitazone indeed increased the protein level of type Ⅱ collagen in a dose-dependent manner with a peak at 1 μM (Fig. 4). These results suggest that the PPAR-γ agonist, rosiglitazone, is a strong inducer of TGF-β and type Ⅱ collagen.
Fig. 4.
Chondrogenic effect according to concentration of rosiglitazone. hBM-MSCs in HA gel were cultured with 0, 0.1, 1, 5, or 10 μM of rosiglitazone in chondrogenic media for 14 days. The protein level of TGF-β and type Ⅱ collagen also increased in a dose-dependent manner with a peak at 1 μM. ∗P < 0.05 versus control. †P < 0.05 versus control.
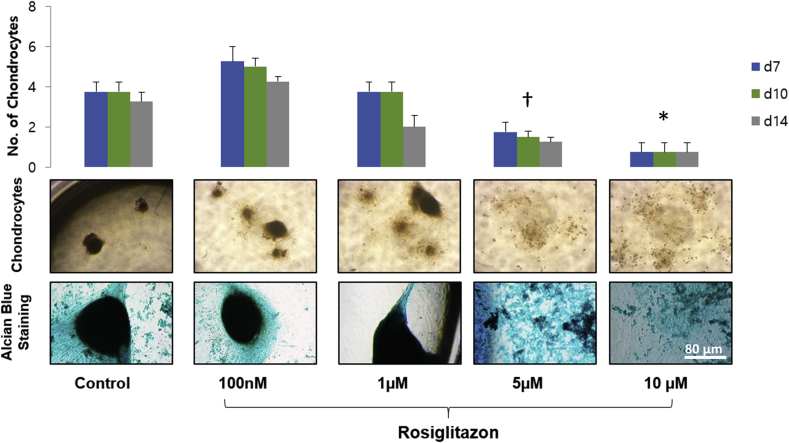
We examined the effect of rosiglitazone on the production of chondrocyte spheroids by Alcian blue staining (Fig. 5). Because of HA gel is also stained by Alcian blue, HA gel was not used in this experiment. hBM-MSCs on plastic walls in chondrogenic medium were treated with 0, 0.1, 1, 5, or 10 μM of rosiglitazone for 14 days. hBM-MSCs with 0.1 μM rosiglitazone generated significantly more chondrocyte spheroids than the control group (without rosiglitazone). However, treatment with more than 1 μM of rosiglitazone inhibited the production of chondrocyte spheroids.
Fig. 5.
The production of chondrocyte according to concentration of rosiglitazone. The production of chondrocyte was quantified by number of spheroids stained by Alcian blue. hBM-MSCs on 96 well plate in chondrogenic medium were treated with 0, 0.1, 1, 5, or 10 μM of rosiglitazone for 14 days. hBM-MSCs with 0.1 μM rosiglitazone generated significantly more chondrocyte spheroids than the control group (without rosiglitazone). More than 1 μM of rosiglitazone was reduced the production of chondrocyte spheroids, compared to the results of the control group. ‡P < 0.05 versus control. ∗P < 0.05 versus control.
3.4. Treatment with a PPAR-γ agonist enhances the production of adipocytes
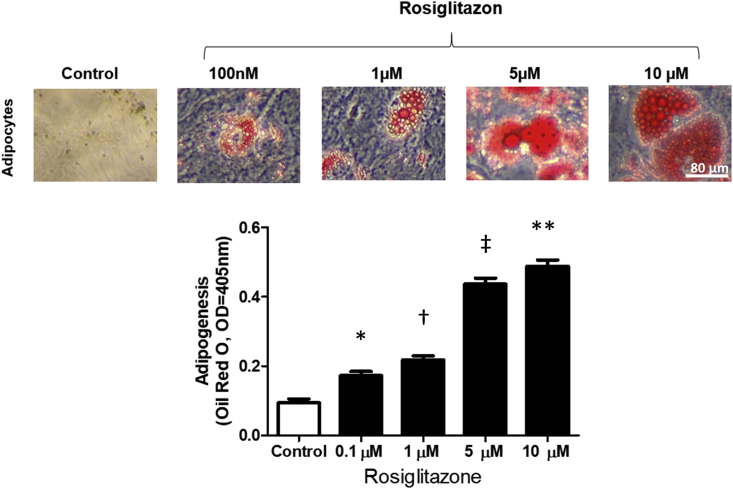
In order to determine the effect of rosiglitazone on adipogenesis, hBM-MSCs on plastic walls in adipogenic medium were treated with 0, 0.1, 1, 5, or 10 μM of rosiglitazone for 14 days. Without rosiglitazone, only a small number of adipocytes were generated, but with rosiglitazone, the production of adipocytes increased in a dose-dependent manner with a peak at 10 μM (Fig. 6). This data shows clearly that rosiglitazone exerts a strong adipogenic effect by enhancing the formation of functional adipocytes.
Fig. 6.
Adipogenic effect of PPAR-δ. hBM-MSCs in adipogenic medium were treated with 0, 0.1, 1, 5, or 10 μM of rosiglitazone for 14 days. The lipid droplets of adipocytes increased in a dose-dependent manner with a peak at 10 μM. ∗P < 0.05 versus control. †P < 0.001 versus control. ‡P < 0.001 versus control. ∗∗P < 0.05 versus control.
4. Discussion
There are various differences between the HA gels available (Table 1, from package insert). The difference between the HA gels was felt to influence the differentiation of the MSCs into Type Ⅱ collagen-producing chondrocyte, and this influence was investigated. Type Ⅱ collagen was expressed and enhanced with all HA gels tested except Supartz® in day 14. Type Ⅱ collagen expression, which is expected as a marker of active, functional chondrocytes, has been noted previously in day 14 [27]. The lack of expression with Supartz® is theorized to be secondary to the lower molecular weight (Table 1) which allowed cells to settle into the gel immediately upon application. The cells settling into the gel did not allow for support from the chondrogenic media for the functional maturation of the spheroid. Other possibilities include the combination of the lower concentration of HA and the structure.
Table 1.
Differences between the hyaluronic acid gels.
| Euflexaa® | Synvisc® | Orthovisc® | Supartz® | |
|---|---|---|---|---|
| Distributor (Manufacturer) | Ferring Pharmaceutical Inc. (Bio-Technology Group) | Sanofi-aventis U.S. LLC (Sanofi) | DePuy Mitek, Inc. (Anika Therapeutics Inc.) | Bioventus LLC (Seikagaku Co.) |
| HA source | Bacterial cells | Avian (Rooster combs) | Bacterial cells | Avian (Rooster combs) |
| Molecular Weight (dalton) | 2.4–3.6 M | 6 M (Average) | 1.0–2.9 M | 0.62–1.17 M |
| HA Concentration | 1% sodium Hyaluronate | 0.8% sodium Hyaluronate | 1.5% sodium Hyaluronate | 1% sodium Hyaluronate |
| HA Structure | Single-chain | Cross-linked | Single-chain | Single-chain |
Traditionally, cell culture of the chondrocyte has been performed using the spin down method [28]. Unfortunately, this represents a two-dimensional culture and does not result in the appropriate environment for spheroid formation. Therefore, a cell mass culture using HA gels was developed by our authors. Type Ⅰ collagen was only expressed in MSCs (Fig. 1a and b). This is to be expected as Type Ⅰ collagen does not express itself in the early stages of chondrogenesis [29].
MMP–13 is traditionally thought to be a degradative enzyme to cartilage [30]. However, it was highly expressed at day 14 in our study. This is in accord with the observation of Salinas et al. who clearly demonstrated increased MMP–13 expression by hMSCs between day 7 and 14 [31]. MMP–13 expression is theorized to have increased expression in HA gel samples secondary to the increased spheroid formation in both number and size (See Suppl. 2).
Our study demonstrated a close relation between PPAR-γ and PPAR-δ. PPAR-δ agonist (GW0742) decreases the expression of PPAR-γ and enhanced the expression of type Ⅱ collagen. In inflammation circumstances, the property of PPAR-δ agonist which inhibit PPAR-γ expression was maintained. The negative correlation of PPAR-γ and type Ⅱ collagen was also shown. Robert et al. reported the contrasting roles of PPAR-γ and PPAR-δ in regulating metabolism in white adipose tissue [32]. This data suggested that PPAR-δ inhibits the expression of PPAR-γ in chondrogenesis. The importance of PPAR-γ on chondrogenesis has been well reported. Shao et al. reported the presence of PPAR isoforms in growth plate cartilage [20]. PPAR-γ knockout mice exhibited spontaneous osteoarthritis [22]. Deletion of PPAR-γ caused abnormal endochondral ossification and impairment of cartilage growth [21]. These studies show that PPAR-γ is an important regulatory factor for osteogenesis and chondrogenesis. However, PPAR-γ has a stronger pro-adipogenic effect than it does a chondrogenic effect. This property makes PPAR-γ an improper candidate for chondrogenic enhancement.
Osteoarthritis synovial fluid activates the inflammation cascade, and inflammation conditions inhibit chondrogenesis [33]. When comparing the expression of type Ⅱ collagen with synovial fluid (Fig. 3: chondrogenic media + 50% synovial fluid + 1 μM rosiglitazone) and without synovial fluid (Fig. 4: chondrogenic media + 1 μM rosiglitazone), there is no significant change in the expression of type Ⅱ collagen. It was shown that the chondrogenic effect of rosiglitazone was not affected by inflammation circumstance. The anti-inflammatory effect of PPAR-γ agonists including rosiglitazone has been reported [19]. This study suggests a considerable anti-inflammatory effect of PPAR-γ agonists. PPAR-γ/δ agonist shows synergistic effect with HA (Fig. 3). Synergistic effect of PPAR-δ agonist and HA was already report [23] but, synergistic effect of PPAR-γ and HA have not been reported yet. This novel data was observed in expression of type Ⅱ collagen and similar synergistic effect was shown between PPAR-δ and HA.
Rosiglitazone (PPAR-γ agonist) enhances the expression of type Ⅱ collagen and TGF-β. However, rosiglitazone at a concentration of greater than 5 μM decreases the enhancement effect of type Ⅱ collagen and TGF-β expression. The expression of PPAR-γ has a negative correlation with the expression of type Ⅱ collagen under PPAR-δ treatment (Fig. 2). These results suggest that a PPAR-γ agonist is not a good chondrogenesis enhancer. It shows different concentration of rosiglitazone to maximize the efficacy of chondrogenesis between formation of chondrocyte spheroid and the expression of type Ⅱ collagen. Formation of chondrocyte spheroid and the expression of type Ⅱ collagen present different properties of chondrogenesis. This hypothesis may explains the reason why those two are different in proper concentration. PPAR-δ agonist inhibits the expression of PPAR-γ. This property of PPAR-δ maintains a lower expression of PPAR-γ, and a PPAR-γ agonist shows a chondrogenic effect at lower concentrations. Our previous study reported that a PPAR-δ agonist has a significant chondrogenic effect [23]. PPAR-γ regulated by PPAR-δ gives a clue to the mechanism of chondrogenesis. Additional research such as analysis of siRNA and administration of PPAR-γ/δ agonist is necessary.
PPAR-γ agonist stimulates adipogenesis and inhibits osteogenesis [24]. This pro-adipogenic effect was enhanced with increasing concentrations of PPAR-γ agonist (Fig. 6). A PPAR-γ agonist inhibits chondrogenesis of MSCs under GW0742 treatment conditions. The anti-chondrogenic effect was enhanced as the PPAR-γ agonist concentration increased. The number of chondrocyte spheroids shows a similar trend to the expression of TGF-β. TGF-β plays an important role in chondrogenesis [23]. This result support hypothesis that PPAR regulates chondrogenesis through TGF-β. The influence of PPAR-γ agonists on chondrocyte and adipocyte spheroid formation in vitro has not been reported. Our study shows the anti-chondrogenic effect and pro-adipogenic effect of PPAR-γ agonist in vitro.
In limitation, whether synergistic effects are affected by inflammation circumstance were not identified. The synergistic effect of HA and PPAR-δ in the expression of type Ⅱ collagen was reported [25]. Some author demonstrated that the function of PPAR-δ agonist is affected by inflammation circumstance [23]. It is a remarkably interesting question whether synergistic effect is influenced by inflammation circumstance. Further studies are required to investigate the synergistic effect of PPAR-δ.
5. Conclusions
In conclusion, HA gel in 3-D culture facilitates the proliferation and differentiation of hMSCs into Type II chondrocytes. Differences in the clinically available HA gels, such as in their source, molecular weight, concentration and structure, may be responsible for the functional changes of hMSC differentiation in this 3-D culture system. Furthermore, various properties of PPAR-γ in chondrogenesis were revealed. PPAR-γ has a considerable anti-inflammatory effect and a strong pro-adipogenic effect, which inhibits the chondrogenic effect. PPAR-γ is regulated by PPAR-δ and shows a chondrogenic effect at lower concentrations. This study suggested that PPAR-γ and PPAR-δ are key regulatory factors of chondrogenesis.
Declarations of Competing Interest
None.
Funding
The authors J.H. is supported by a 2-Year Research Grant of Pusan National University, Republic of Korea.
Ethical approval
This study was reviewed and approved by the Institutional Review Board of the University of Toledo.
Informed consent
After obtaining informed consent from the patients, discarded synovial fluids were obtained.
Author contributions
Dong Hyun Kim, Dong Hwan Kim; collection and/or assembly of data, data analysis and interpretation, manuscript writing. Bruce E. Heck, Michael Shaffer; collection and/or assembly of data. Keon Hee Yoo; conception and design, data analysis and interpretation. Jin Hur; conception and design, manuscript writing, data analysis and interpretation, final approval of manuscript.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2020.07.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
fig s1.
fig s2.
fig s3.
References
- 1.Brooks P.M. Impact of osteoarthritis on individuals and society: how much disability? Social consequences and health economic implications. Curr Opin Rheumatol. 2002;14(5):573–577. doi: 10.1097/00002281-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Freyria A.M., Mallein-Gerin F. Chondrocytes or adult stem cells for cartilage repair: the indisputable role of growth factors. Injury. 2012;43(3):259–265. doi: 10.1016/j.injury.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 3.Sophia Fox A.J., Bedi A., Rodeo S.A. The basic science of articular cartilage: structure, composition, and function. Sport Health. 2009;1(6):461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frean S.P., Abraham L.A., Lees P. In vitro stimulation of equine articular cartilage proteoglycan synthesis by hyaluronan and carprofen. Res Vet Sci. 1999;67(2):183–190. doi: 10.1053/rvsc.1999.0328. [DOI] [PubMed] [Google Scholar]
- 5.Erickson I.E., Huang A.H., Sengupta S., Kestle S., Burdick J.A., Mauck R.L. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis Cartilage. 2009;17(12):1639–1648. doi: 10.1016/j.joca.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung C., Mesa J., Randolph M.A., Yaremchuk M., Burdick J.A. Influence of gel properties on neocartilage formation by auricular chondrocytes photoencapsulated in hyaluronic acid networks. J Biomed Mater Res. 2006;77(3):518–525. doi: 10.1002/jbm.a.30660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billinghurst R.C., Dahlberg L., Ionescu M., Reiner A., Bourne R., Rorabeck C. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99(7):1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldring S.R., Goldring M.B. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;427(Suppl):S27–S36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 9.Loeser R.F. Molecular mechanisms of cartilage destruction in osteoarthritis. J Musculoskelet Neuronal Interact. 2008;8(4):303–306. [PubMed] [Google Scholar]
- 10.Goldring M.B., Goldring S.R. Osteoarthritis. J Cell Physiol. 2007;213(3):626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 11.Campo G.M., Avenoso A., Campo S., Ferlazzo A.M., Altavilla D., Calatroni A. Efficacy of treatment with glycosaminoglycans on experimental collagen-induced arthritis in rats. Arthritis Res Ther. 2003;5(3):R122–R131. doi: 10.1186/ar748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grishko V., Xu M., Ho R., Mates A., Watson S., Kim J.T. Effects of hyaluronic acid on mitochondrial function and mitochondria-driven apoptosis following oxidative stress in human chondrocytes. J Biol Chem. 2009;284(14):9132–9139. doi: 10.1074/jbc.M804178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki A., Sasaki K., Konttinen Y.T., Santavirta S., Takahara M., Takei H. Hyaluronate inhibits the interleukin-1 beta-induced expression of matrix metalloproteinase (MMP)-1 and MMP-3 in human synovial cells. Tohoku J Exp Med. 2004;204(2):99–107. doi: 10.1620/tjem.204.99. [DOI] [PubMed] [Google Scholar]
- 14.Lo G.H., LaValley M., McAlindon T., Felson D.T. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. J Am Med Assoc. 2003;290(23):3115–3121. doi: 10.1001/jama.290.23.3115. [DOI] [PubMed] [Google Scholar]
- 15.Miller L.E., Block J.E. US-approved intra-articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: systematic Review and meta-analysis of randomized, saline-controlled trials. Clin Med Insights Arthritis Musculoskelet Disord. 2013;6:57–63. doi: 10.4137/CMAMD.S12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berenbaum F., Grifka J., Cazzaniga S., D'Amato M., Giacovelli G., Chevalier X. A randomised, double-blind, controlled trial comparing two intra-articular hyaluronic acid preparations differing by their molecular weight in symptomatic knee osteoarthritis. Ann Rheum Dis. 2012;71(9):1454–1460. doi: 10.1136/annrheumdis-2011-200972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kota B.P., Huang T.H., Roufogalis B.D. An overview on biological mechanisms of PPARs. Pharmacol Res. 2005;51(2):85–94. doi: 10.1016/j.phrs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Schug T.T., Berry D.C., Shaw N.S., Travis S.N., Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129(4):723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paukkeri E.L., Leppanen T., Lindholm M., Yam M.F., Asmawi M.Z., Kolmonen A. Anti-inflammatory properties of a dual PPARgamma/alpha agonist muraglitazar in in vitro and in vivo models. Arthritis Res Ther. 2013;15(2):R51. doi: 10.1186/ar4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao Y.Y., Wang L., Hicks D.G., Tarr S., Ballock R.T. Expression and activation of peroxisome proliferator-activated receptors in growth plate chondrocytes. J Orthop Res. 2005;23(5):1139–1145. doi: 10.1016/j.orthres.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Monemdjou R., Vasheghani F., Fahmi H., Perez G., Blati M., Taniguchi N. Association of cartilage-specific deletion of peroxisome proliferator-activated receptor gamma with abnormal endochondral ossification and impaired cartilage growth and development in a murine model. Arthritis Rheum. 2012;64(5):1551–1561. doi: 10.1002/art.33490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasheghani F., Monemdjou R., Fahmi H., Zhang Y., Perez G., Blati M. Adult cartilage-specific peroxisome proliferator-activated receptor gamma knockout mice exhibit the spontaneous osteoarthritis phenotype. Am J Pathol. 2013;182(4):1099–1106. doi: 10.1016/j.ajpath.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Heck B.E., Park J.J., Makani V., Kim E.C., Kim D.H. PPAR-delta agonist with mesenchymal stem cells induces type II collagen-producing chondrocytes in human arthritic synovial fluid. Cell Transplant. 2017;26(8):1405–1417. doi: 10.1177/0963689717720278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lecka-Czernik B., Moerman E.J., Grant D.F., Lehmann J.M., Manolagas S.C., Jilka R.L. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143(6):2376–2384. doi: 10.1210/endo.143.6.8834. [DOI] [PubMed] [Google Scholar]
- 25.Kim D.H., Liu J., Bhat S., Benedict G., Lecka-Czernik B., Peterson S.J. Peroxisome proliferator-activated receptor delta agonist attenuates nicotine suppression effect on human mesenchymal stem cell-derived osteogenesis and involves increased expression of heme oxygenase-1. J Bone Miner Metabol. 2013;31(1):44–52. doi: 10.1007/s00774-012-0382-0. [DOI] [PubMed] [Google Scholar]
- 26.Cuzzocrea S., Mazzon E., Dugo L., Patel N.S., Serraino I., Di Paola R. Reduction in the evolution of murine type II collagen-induced arthritis by treatment with rosiglitazone, a ligand of the peroxisome proliferator-activated receptor gamma. Arthritis Rheum. 2003;48(12):3544–3556. doi: 10.1002/art.11351. [DOI] [PubMed] [Google Scholar]
- 27.Altaf F.M., Hering T.M., Kazmi N.H., Yoo J.U., Johnstone B. Ascorbate-enhanced chondrogenesis of ATDC5 cells. Eur Cell Mater. 2006;12:64–69. doi: 10.22203/ecm.v012a08. discussion 9-70. [DOI] [PubMed] [Google Scholar]
- 28.Solchaga L.A., Penick K.J., Welter J.F. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: tips and tricks. Methods Mol Biol. 2011;698:253–278. doi: 10.1007/978-1-60761-999-4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marlovits S., Hombauer M., Truppe M., Vecsei V., Schlegel W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J Bone Joint Surg Br. 2004;86(2):286–295. doi: 10.1302/0301-620x.86b2.14918. [DOI] [PubMed] [Google Scholar]
- 30.Wang M., Sampson E.R., Jin H., Li J., Ke Q.H., Im H.J. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15(1):R5. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salinas C.N., Anseth K.S. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials. 2008;29(15):2370–2377. doi: 10.1016/j.biomaterials.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts L.D., Murray A.J., Menassa D., Ashmore T., Nicholls A.W., Griffin J.L. The contrasting roles of PPAR delta and PPAR gamma in regulating the metabolic switch between oxidation and storage of fats in white adipose tissue. Genome Biol. 2011;12(8):R75. doi: 10.1186/gb-2011-12-8-r75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoff P., Buttgereit F., Burmester G.R., Jakstadt M., Gaber T., Andreas K. Osteoarthritis synovial fluid activates pro-inflammatory cytokines in primary human chondrocytes. Int Orthop. 2013;37(1):145–151. doi: 10.1007/s00264-012-1724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]