Figure 5.
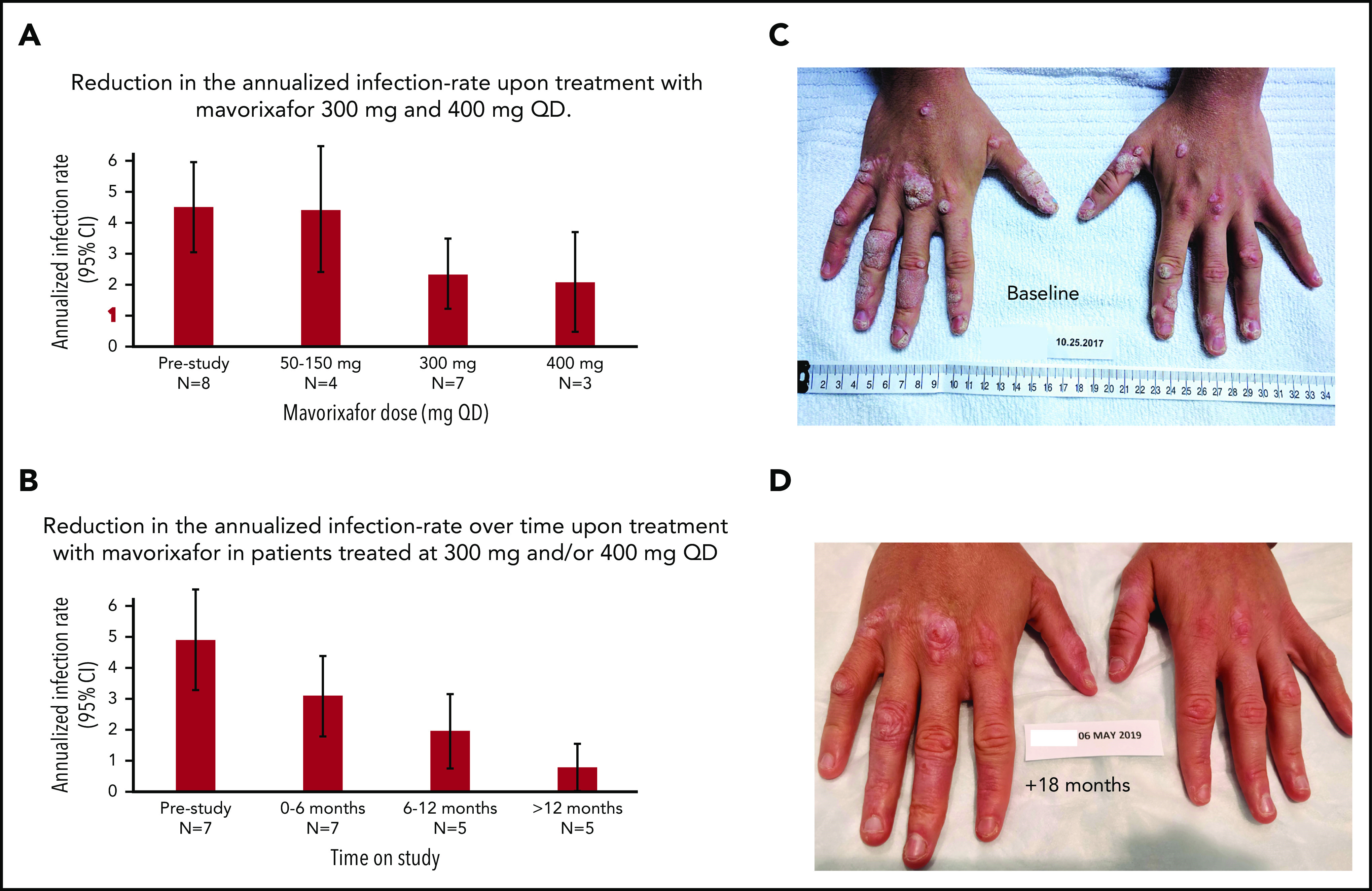
Clinical response to treatment. (A) Reduction in the annualized infection rate upon treatment with mavorixafor, 300 mg and 400 mg once daily. Reduction in the annualized infection rate upon treatment with mavorixafor, 300 mg and 400 mg once daily, compared with the 12 months prior and to lower doses of mavorixafor (50 to 150 mg once daily). The retrospective yearly infection rate in the 12 months prior to the trial was calculated based on the safety population (n = 8). The yearly infection rate is derived from the sum of the number of infection events for each patient divided by the total exposure time (in years). (B) Reduction in the annualized infection rate over time upon treatment with mavorixafor in patients treated at 300 mg and/or 400 mg once daily. Yearly infection rates compared with the 12 months prior to the study. The retrospective yearly infection rate in the 12 months prior to the trial was calculated based on the patients treated up to ≥300 mg (n = 7). The yearly infection rate at each treatment period is derived from the sum of the number of infection events for each patient divided by the exposure time (in years) at each time period. (C-D) Significant reduction in the number of cutaneous warts during long-term once-daily mavorixafor treatment. Patient P6 was treated with increasing doses of mavorixafor alone for a total of 18 months (including 14 months at 400 mg once daily). The patient was not given imiquimod or other treatments for HPV. (C) Warts at baseline. (D) Warts 18 months later, after 14 months at 400 mg. A significant decrease in wart burden could be seen after 6 months on treatment.
