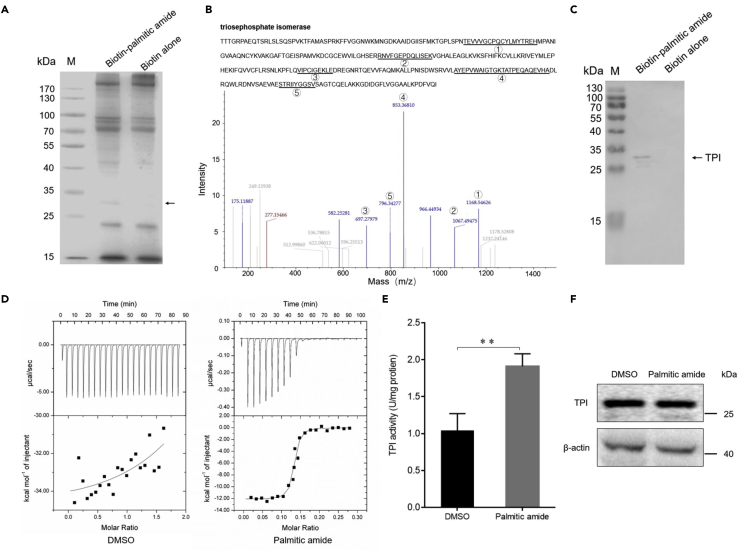
Figure 5.
Palmitic amide interacted with the TPI protein and increased the TPI activity
(A) Proteins interacted with palmitic amide. Shrimp hemocytes were lysed and then incubated with biotin-labeled palmitic amide. The proteins were analyzed by SDS-PAGE with Coomassie blue staining. Dynabeads alone was used as a control. The arrow indicated the differential protein. M, protein marker.
(B) Identification of the protein bound to palmitic amide by mass spectrometry. The protein was identified to be triosephosphate isomerase. The matched peptides were indicated with underlines and numbers.
(C) Western blot analysis of the proteins interacted with palmitic amide. The arrow indicated the TPI. M, protein marker.
(D) Thermodynamic characterization of the interaction between palmitic amide and TPI protein. The purified recombinant TPI protein (100 μM) and incubated with palmitic amide (1 mM), followed by isothermal titration calorimetry (ITC) analysis. DMSO was used as a control.
(E) Impact of palmitic amide on TPI activity in shrimp. Shrimp were injected with palmitic amide (100 μM). Twenty-four hours later, the activity of TPI of hemocytes was examined. DMSO was used as a control. The error bars denoted the means ± standard deviations of three independent experiments (∗∗p < 0.01).
(F) Influence of palmitic amide on the expression of TPI in shrimp. Shrimp were injected with palmitic amide (100 μM) or DMSO. Twenty-four hours later, the expression level of TPI in the hemocytes of shrimp was examined by Western blot analysis. β-actin was used as a control.