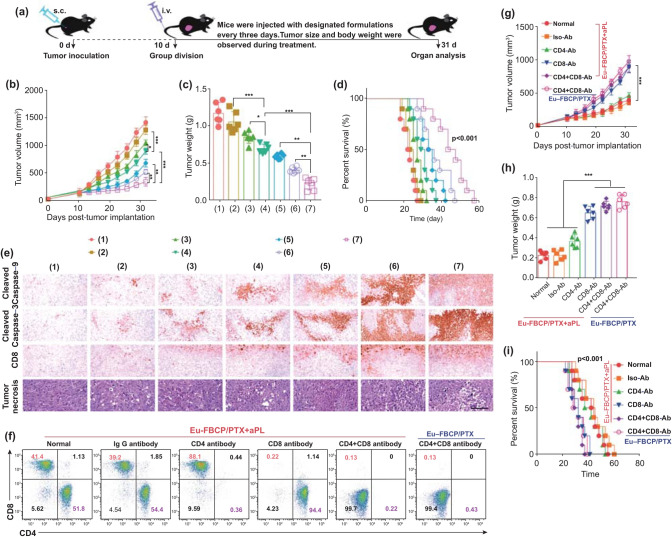
Fig. 7.
In vivo antitumor efficacies of Eu-FBCP/PTX and Eu-s/PTX in the absence (inducing chemical ICD) and presence (boosting immuno-antitumor activity) of aPL (*p < 0.05, **p < 0.01, and ***p < 0.001). a Schematic of the experimental schedules for in vivo antitumor studies with different treatments (1: PBS, 2: free PTX, 3: Eu-s/PTX, 4: Eu-FBCP/PTX, 5: aPL, 6: Eu-s/PTX + aPL, and 7: Eu-FBCP/PTX + aPL). b, c Time profiles of tumor sizes and final weights of tumors collected from the treated mice (1–7; six mice per group). d Survival curves of the treated mice (1–7; ten mice per group). e Histopathological and immunohistochemical expression of cleaved caspase-9, cleaved caspase-3, CD8+, and tumor necrosis factor in tumor sections obtained from the treated mice (1–7; six mice per group). f Immune cell generation after pretreatments with PBS (normal), IgG antibody (Iso-Ab), CD4 antibody (CD4-Ab), CD8 antibody (CD8-Ab), and CD8 + CD4 antibody (CD4 + CD8-Ab) to construct an immunocompromized MC-38 tumor-bearing mouse model. g, h Time profiles of tumor sizes and final weights of tumors collected from the immunocompromized mice singly treated with Eu-FBCP/PTX + aPL or Eu-FBCP/PTX (six mice per group). i Survival curves of the immunocompromized mice singly treated with Eu-FBCP/PTX + aPL or Eu-FBCP/PTX (ten mice per group)