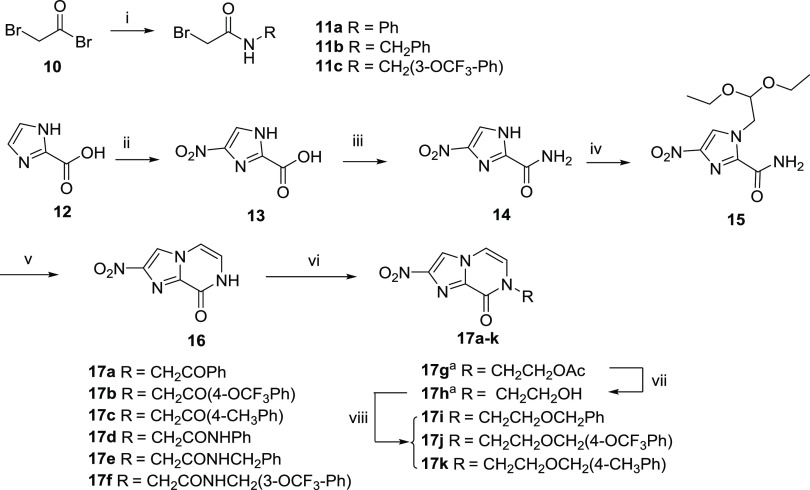
Scheme 1. Synthesis of Nitroimidazopyrazinones 17a–k.
(i) Various amines, TEA, DCM, 0 °C → rt; (ii) H2SO4/HNO3, 60 °C; (iii) oxalyl chloride, catalytic DMF, DCM, 0 °C → rt, then concentrated NH4OH, 0 °C → rt; (iv) bromoacetaldehyde diethyl acetal, K2CO3, μW 180 °C; (v) 2 M HCl/1,4-dioxane, μW 120 °C; (vi) various alkyl bromides, Cs2CO3 or K2CO3, DMF, μW 80–100 °C; (vii) K2CO3, MeOH, rt; (viii) various benzyl bromides, NaH, DMF, 0 °C → rt. aReported in a previous study.26