Abstract
Objective.—
To determine whether patients with vestibular migraine are more likely to suffer from an occipital headache than patients with migraine without vestibular symptoms.
Background.—
Vestibular migraine is an underdiagnosed disorder in which migraine is associated with vestibular symptoms. Anatomical evidence and symptomatology hint at the involvement of brain structures in the posterior fossa (back of the head location). We hypothesized that vestibular migraine patients are more likely than migraineurs without vestibular symptoms to experience headaches located in the back of the head, that is, occipital headaches.
Methods.—
A retrospective cross-sectional study was conducted at the University of Iowa Hospital and Clinics. Chart analysis of 169 patients was performed. The primary outcome was the location of the headache in vestibular migraine patients and migraineurs without vestibular symptoms. The secondary outcomes included the association of vestibular migraine with gender, age at onset of headache, age at onset of vestibular symptoms (such as vertigo, head motion-induced dizziness), aura, motion sickness, other associated symptoms, family history of headaches, and family history of motion sickness.
Results.—
In vestibular migraine group, 45/103 (44%) had occipital location for their headaches vs 12/66 (18%) in migraine patients without vestibular symptoms, for an odd’s ratio of 3.5 (95% CI = 1.7–7.2, P < .001). Additionally, the age at onset of headache was greater in the vestibular migraine group (28 ± 12 vs 18 ± 9 years, P < .001) and motion sickness was more common (41/98 (42%) in the vestibular migraine group, 1/64 (2%) in the migraine without vestibular symptoms group, P < .001).
Conclusions.—
This study suggests that patients with vestibular migraine are more likely to have occipital headaches than patients with migraine without vestibular symptoms. Our data support the initiation of a prospective study to determine whether a patient presenting with occipital headaches, with late onset of age of headache, and with a history of motion sickness is at an increased risk for the possible development of vestibular migraine.
Keywords: vestibular migraine, migraine, headache location, occipital, vertigo, dizziness
INTRODUCTION
Vestibular migraine (VM) is a significantly under-recognized disorder and needs better characterization to improve its diagnosis.1–10 VM is considered a type of migraine where patients suffer from headaches and vestibular symptoms such as vertigo or head motion-induced dizziness.11,12 VM diagnostic criteria were first established 20019 and it is currently diagnosed using the criteria of the International Classifications of Headache Disorders (ICHD-3).13 The reported incidence of VM in the United States is 1%–3% of the adult population9,14 and 10%–30% of patients seen in headache and dizziness clinics.11,15 Although it is one of the most common presenting conditions to primary care, otology, and neurology,11 it remains significantly underdiagnosed, with VM ultimately diagnosed in 20.2% of the population studied based on specialist evaluations, although it was only suspected in 1.8%.5 It is estimated that 80% of VM patients do not carry the correct diagnosis.9 Given this large gap in VM recognition, any attempt to improve its characterization has the potential to significantly improve recognition, diagnosis, and clinical outcome.
One way to improve the recognition of VM is to better characterize its clinical presentation. The anatomical structures that have been implicated in generating vestibular symptoms (such as vertigo or head motion-induced dizziness) are the components of the vestibular system, whose fibers project from the inner ear to the brainstem and cerebellum, that is. the posterior fossa, or back of the head.16 Additional clues implicating the posterior fossa structures in the generation of vestibular symptoms come from pathologies that cause vestibular symptomatology such as vertebral artery dissection, and that also induce pain in the back of the head. Based on this anatomical and pathological information, we hypothesized that VM patients experience more pain in the occipital region (back of the head) than do migraine patients who do not suffer from vestibular symptoms (henceforth referred to here as M patients).
In this work, we will test the null hypothesis that there is no difference in the proportion of occipital headaches between the VM and M groups. Although no definitive conclusions can be drawn from a retrospective chart analysis, the goal of the present study is to provide a first assessment of the validity of our hypothesis, as a test of whether a prospective study is warranted. Specifically, we aimed to characterize differences in the headache location in VM vs M patients.
METHODS
This study was approved by the University of Iowa Institutional IRB (#201404723), and informed consent was waived.
We conducted a cross-sectional retrospective study, where patient charts were retrospectively selected from patients seen within the University of Iowa Hospitals and Clinics (Iowa City), at 2 of our clinics, Balance Disorders, and Headache Clinics, between January 2008 and April 2014.
The Balance Disorders Clinic evaluates patients manifesting with balance, vertigo, or dizziness, and a separate Headache Clinic evaluates headache patients. A neuro-otologist (DF) saw all patients coming to the Balance Disorders Clinic in person. Those patients filled out a dizziness questionnaire (designed by DF) containing detailed questions about vertigo/dizziness/balance and about the presence of headache, its location, and its characteristics. A headache specialist (AR) saw patients coming to the headache clinic in person. Those patients filled out a headache questionnaire (designed by AR) at the time of their visit. Among detailed questions about headache, it also asked about the presence of symptoms such as vertigo or dizziness, and about headache location.
Questionnaires were filled out before the doctor was seen in order to minimize bias. Importantly, the questionnaires were designed to help the physicians in their diagnosis rather than specifically for the purpose of this study. A final diagnosis was made based both on the questionnaire and the information found in the charts. Thus, some charts were missing some information to properly diagnose VM. For example, if a migraine patient had vestibular symptoms (such as vertigo), but their chart did not have sufficient details to diagnose VM, then such a chart was not included. As a result, only 2 groups of patients were included: VM patients, who fulfilled ICHD3 criteria13 and patients with migraines without any vestibular symptoms (M group), also based on ICHD3 criteria.13
A single list of migraine patients seen at both our Balance Disorders and Headache Clinics was generated by our University coding personnel, including all patients that presented with migraine, independently of vestibular symptoms. The research team was given 1 single list of more than 1500 names of migraine patients from both the Balance Disorders and Headache Clinics, and one of us (SO) randomly/blindly selected 200 charts from that pool; our sample was a simple random sample (see Fig. 1).
Fig. 1.—
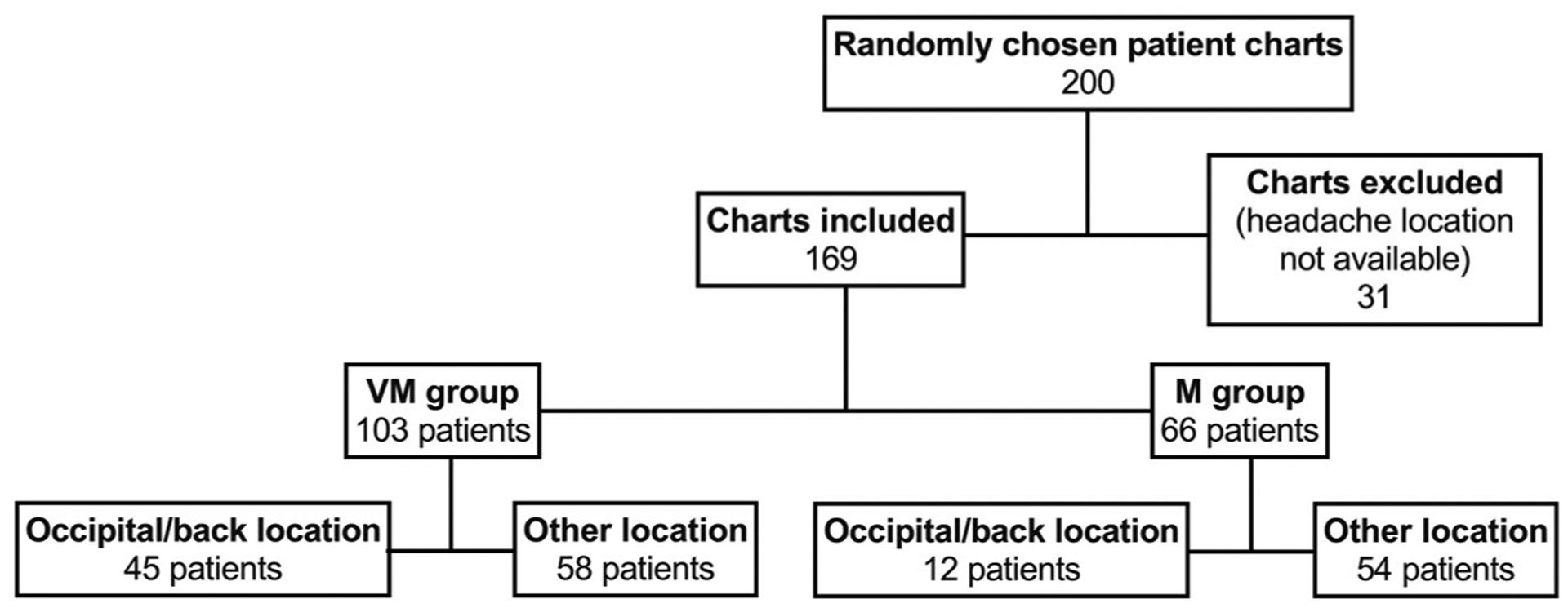
Illustration of selection of patient charts for this study. Patients were seen at a clinic assessing patients for issues with dizziness and balance or at a clinic assessing patients for headache.
Identifying information was removed and all notes were assigned a numeric identifier that cannot be traced back to the original chart. Retrospectively, diagnostic criteria were applied to the charts to distinguish between VM and M based on the ICHD3 classification.17 The charts were reviewed for the location of the headache, and any chart missing this information was excluded from the study (Fig. 1). The headache location was assessed using an open-ended question in the dizziness questionnaire, and a multiple-choice question in the headache questionnaire. Any headache described as being located “in the back of the head” or using similar wording was then coded as occipital/back location for the purpose of this study. Neck pain was distinguished from occipital headache. The occipital/back location classification was used both when it was the only headache location and when it was named among other locations. However, a headache characterized as either holocephalic (diffuse throughout the whole head) or affecting an entire side of the head without specific mention of the back of the head was not classified as occipital/back location.
The charts were also reviewed for the following additional information. For all patients, we collected age of onset of headache and for those who fulfilled VM criteria, we collected the age of onset of vestibular symptoms; other collected data were gender; frequency of headaches; symptoms associated with the headache (such as nausea, vomiting, photophobia, phonophobia, and visual aura); symptoms associated with vestibular symptoms (such as photophobia, phonophobia, visual aura, headaches); personal history of motion sickness (worded as car sickness in the questionnaire from the Balance Clinic, and penciled-in for patients answering the questionnaire from the Headache Clinic); family history of migraine or headaches, and family history of motion sickness.
In total, 31 charts were excluded from the study. In all cases, this was for failure to report the location of the headache (Fig. 1). Although not all charts contained all the additional information listed above, no others were excluded from the study. The numbers of patients for which specific types of data were missing are provided in the figure legends, both as totals and as a percentage of the number of participants in each group. Notably, the gaps in information are similar for both groups.
Statistical Analysis.—
This study is a primary analysis (a priori) of information obtained from a retrospective chart review for VM and M patients. The number of charts needed for review was determined using sample size calculation online software (https://clincalc.com/stats/samplesize.aspx), using 2 independent groups, dichotomous outcome, events 40% in the VM and 20% in the M group, and with the goal of achieving a power of 0.8 and an alpha value of 0.05. The calculated sample size was 162, and this was raised to 200 to take into account the lack of some data. Our statistician (PTE) repeated the calculations and found similar results using PROC POWER in SAS 9.4, with a sample size of 82 subjects per group to achieve 80% power when testing for a difference between 2 proportions believed to be 20% and 40% at the α = 0.05 level. Descriptive variables are reported as mean ± standard deviation, whereas categorical variables are reported as frequency counts (%) of the patient population within a group. GraphPad Prism software was used for the analysis. The significance of the difference between groups in terms of mean values was evaluated using the 2-sample t- and Mann Whitney tests. Categorical variables were evaluated using Chi-square test. Comparisons between groups were reported as odds ratios (OR) and 95% confidence intervals (CI). A simple linear regression is performed between variable 1 (age of onset of headache) and variable 2 (age of onset of vestibular symptoms) in order to assess their relationship (association). The goodness to fit value (R2) is reported. Statistical significance was assumed at P < .05, and all inferences were 2-sided where appropriate. The radar chart was generated using www.onlinecharttool.com.
RESULTS
Demographics.—
We included 169 patients in this study; 103 patients fulfilled the criteria for classification in the VM group and 66 fulfilled the criteria for classification in the M group. The VM group consisted of 79% females and 21% males (3.7:1 ratio) and the M group consisted of 88% females and 12% males (7.3:1 ratio; Fig. 2a). The clear female predominance is not statistically different between groups (P = .184).
Fig. 2.—
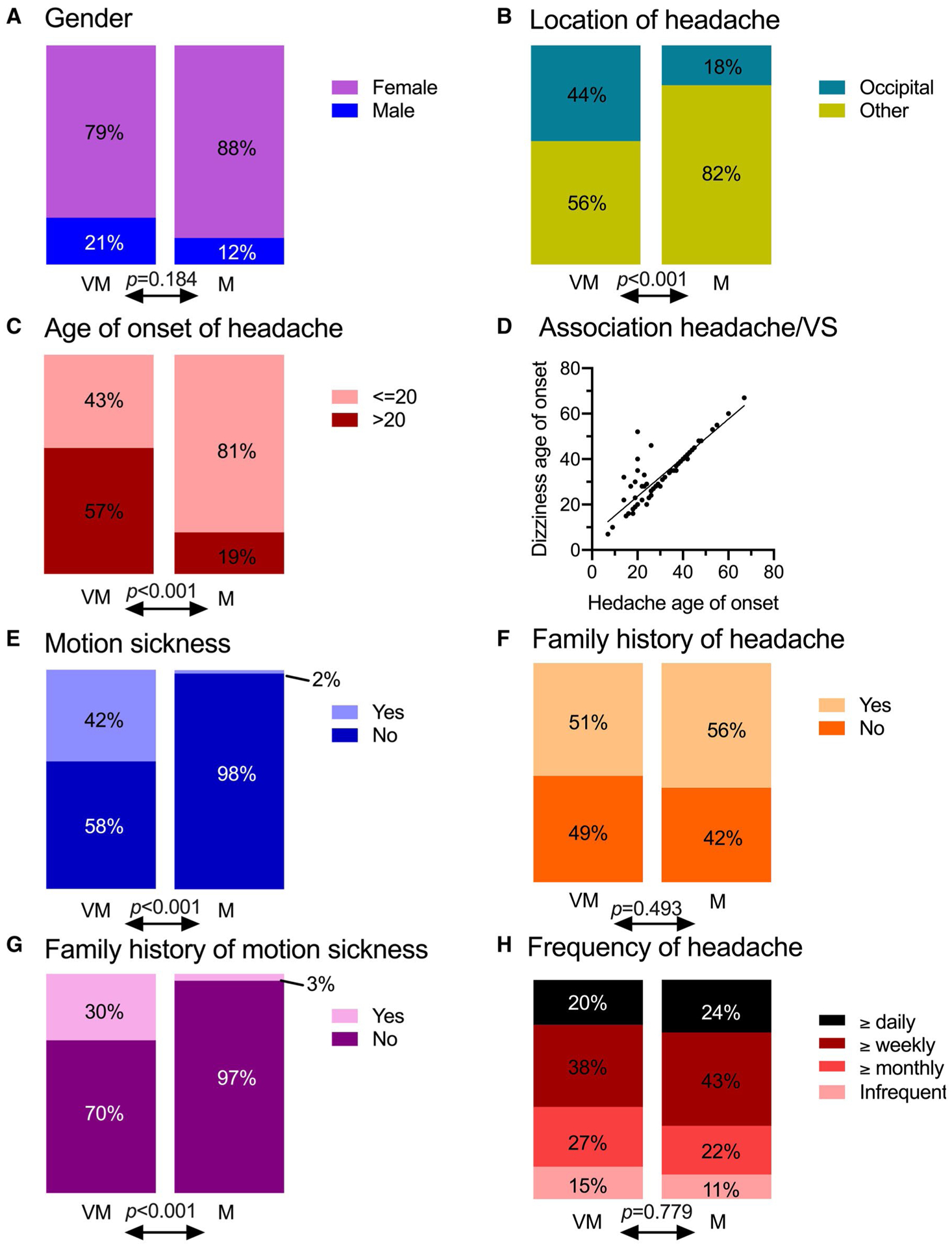
Demographics and symptoms of patients who were divided into the VM and M groups. (A) Gender. (B) Occipital or other location of headache. (C) Age of onset of headache (missing data: 16 for VM patients and 8 for M patients). (D) Association between ages of headache onset and onset of vestibular symptoms (VS) in VM patients (linear regression, slope = 0.851, R2 = 0.763, P < .001). (E) Personal history of motion sickness (missing data: 5 for VM patients and 2 for M patients). (F) Family history of headache (missing data: 2 for VM patients and 1 for M patients). (G) Family history of motion sickness (missing data: 4 for VM patients and 2 for M patients). (H) Frequency of headache (missing data: 18 for VM patients and 12 for M patients). Missing data are not represented in these graphs and were excluded from the statistical analysis. ***P < .001 Chi-square test between groups.
Primary Outcome: Occipital Location of Headache.—
The primary outcome of this study is to gather information about the location of headache in patients of the VM and M groups. In the VM group, 45/103 or 44% of patients were counted positive for occipital/back of head headache, vs 12/66 or 18% in the M group (P < .001, Fig. 2b). In patients suffering from VM, the odd’s ratio OR of having an occipital headache was 3.5 times the odds for patients suffering from M (OR 3.5, 95% confidence interval (CI) of 1.7–7.2). Of note, 53% of VM patients with an occipital headache reported that their headaches were located exclusively in the occipital/back area, whereas only 25% of M patients reported this exclusivity (P = .081).
Secondary Outcomes.—
Age of Onset of Headaches.—
The mean age at headache onset was statistically significantly higher in the VM group (28 ± 12 years old) than in the M group (18 ± 9 years, P < .001, not adjusted for a potential difference in the age of the patients from both clinics at the time of the visit). However, the exact age of onset was reported in only 69% of the charts, and some patients had provided only a vague answer to this question, such as “since childhood” or “as a teenager”. Given the prevalence of this type of answer, we were able to achieve a more inclusive representation of the population by separating the patients into 2 groups with onsets ≤20 years old and >20 years old (Fig. 2c). Among the VM patients, 50/87 (57%) had an onset of headache at over 20 years old, compared to 11/58 (19%) in the M group (P < .001, OR = 5.77, 95% CI 2.62–12.10).
Age of Onset of Vestibular Symptoms.—
The age of onset of vestibular symptoms was 32 ± 12 years old in VM patients; this is statistically significantly later than headache onset (3 years, P = .005, independent t-test). Among the VM patients, the onset of vestibular symptoms after headache onset was reported in 30% of cases, simultaneous onset in 60% of cases, and the occurrence of vestibular symptoms before headache was reported in 10% of cases. These ages of onset of headache and vestibular symptoms are significantly associated (Fig. 2d, linear regression slope = 0.85, R2 = 0.763, P < .001), indicating that vestibular symptoms and headache are likely caused by the same pathology.
Co-Occurrence of Headache and Vestibular Symptoms.—
Among the VM patient group, 46% reported experiencing vestibular symptoms and headaches simultaneously and 4% reported experiencing them independent of one another. The remaining 50% of patients did not provide a clear answer on this topic.
Gender Differences.—
No gender differences in the age of onset of headaches were apparent in the M group (18 ± 8 years for females and 17 ± 12 years for males, P = .914). However, among the VM patients, headache onset was statistically significantly earlier in females, at 26 ± 11 years vs 34 ± 14 years (P = .012). Surprisingly considering the significant statistical association between the age of onset of headache and gender, there was no gender difference in the age of onset of vestibular symptoms (31 ± 12 years for females and 35 ± 13 years for males, P = .158).
Motion Sickness.—
Symptoms of motion sickness since childhood were reported by significantly more patients in the VM group (42%) than in the M group (2%) (Fig. 2e).
Family History.—
There was no difference between the VM and M groups in terms of family history of headache (Fig. 2f). In the VM group, 51% of patients and in the M group 57% of patients reported the presence of headache in family members (P = .49). In the case of motion sickness, however, the difference in family history was significant, with the VM group reporting 30% and the M group only 3% (Fig. 2g, P < .001, OR = 13.48, 95% CI 3.47–58.61).
Headache Frequency.—
There was no difference in the headache frequency between the 2 groups (P = .82). Most patients suffered from weekly headaches (between 1 and 6 per week, Fig. 2h).
Associated Symptoms.—
The medical chart also provided a list of symptoms associated with either the headache and/or vestibular symptoms. Those symptoms are reported in Table 1. Notably, the only difference observed between the VM and M groups related to the symptoms of aura and vomiting. VM patients reported less aura (2%) and less vomiting (24%) than M patients (23%, P < .001, OR = 0.07, 95% CI 0.01–0.27 for aura; 41%, P < .034, OR = 0.46, 95% CI 0.24–0.92 for vomiting).
Table 1.—
Differences Between Features of VM and Migraine Without Vestibular Symptoms
| VM Group # of Patients (% of Patients Who Answered) | Migraine Group # of Patients (% of Patients Who Answered) | P Value* = Significance | ||||||
|---|---|---|---|---|---|---|---|---|
| Total number of patients | 103 | 66 | ||||||
| Gender | ||||||||
| Female | 81 (79%) | 58 (88%) | 0.184 | |||||
| Male | 22 (21%) | 8 (12%) | ||||||
| Age of onset of headache | ||||||||
| Average | 28 ± 12 years old | 17 ± 9 years old | <0.001* | |||||
| ≤20 years old | 37 (43%) | 47 (81%) | <0.001* | |||||
| >20 years old | 50 (57%) | 11 (19%) | ||||||
| Age of onset of vestibular symptoms | ||||||||
| Average | 32 ± 12 years old | N/A | N/A | |||||
| Location of headache | ||||||||
| Only occipital | 24 (23%) | 45 (44%) | 3 (4%) | 12 (18%) | 0.032* | |||
| Occipital + other | 21 (20%) | 9 (14%) | ||||||
| Not occipital | 58 (56%) | 54 (82%) | 0.001* | |||||
| Motion sickness since childhood | ||||||||
| Yes | 41 (42%) | 1 (2%) | 0.001* | |||||
| No | 57 (58%) | 63 (98%) | ||||||
| Family history of headache | ||||||||
| Yes | 52 (51%) | 37 (56%) | 0.599 | |||||
| No | 49 (49%) | 28 (42%) | ||||||
| Family history of motion sickness | ||||||||
| Yes | 30 (30%) | 2 (3%) | 0.001* | |||||
| No | 69 (70%) | 62 (97%) | ||||||
| Frequency of headache | ||||||||
| ≥1/day | 18 (20%) | 13 (24%) | 0.831 | |||||
| <1/day to ≥ 1/week | 33 (38%) | 23 (43%) | ||||||
| <1/week to ≥ 1/ month | 24 (27%) | 12 (22%) | ||||||
| <1/month | 13 (15%) | 6 (11%) | ||||||
| Photophobia | ||||||||
| Yes | 90 (87%) | 62 (94%) | 0.262 | |||||
| No/not reported | 13 (13%) | 4 (6%) | ||||||
| Visual perturbations other than photophobia | ||||||||
| Yes | 10 (10%) | 2 (3%) | 0.179 | |||||
| No/not reported | 93 (90%) | 64 (97%) | ||||||
| Phonophobia | ||||||||
| Yes | 80 (78%) | 59 (89%) | 0.089 | |||||
| No/not reported | 23 (22%) | 7 (11%) | ||||||
| Aura | ||||||||
| Yes | 2 (2%) | 15 (23%) | 0.001* | |||||
| No/not reported | 101 (98%) | 51 (77%) | ||||||
| Nausea | ||||||||
| Yes | 69 (67%) | 53 (80%) | 0.088 | |||||
| No/not reported | 34 (33%) | 13 (20%) | ||||||
| Vomiting | ||||||||
| Yes | 25 (24%) | 27 (41%) | 0.034* | |||||
| No/not reported | 78 (75%) | 39 (59%) | ||||||
Indicates P < .05.
The radar chart shown in Figure 3 illustrates the differences observed between the VM and the M groups.
Fig. 3.—
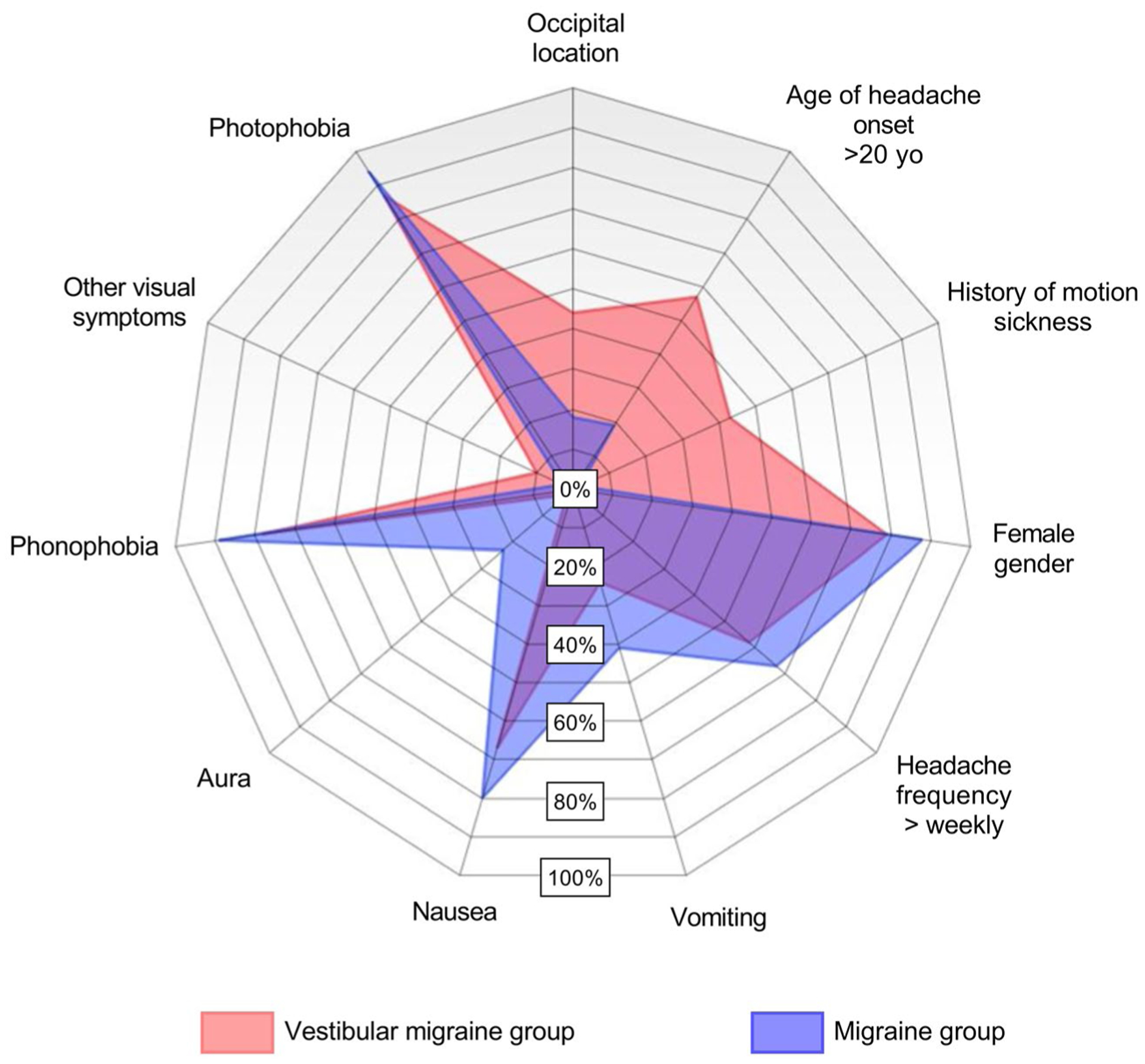
Radar chart showing demographics and symptoms for VM (red) and M (blue) patients. Overlapping populations are purple. Occipital location, age of headache onset, and history of motion sickness are grouped on the top right side of the graph to highlight the main findings from the present study.
DISCUSSION
The primary outcome of this study is the novel finding that VM patients are more likely to have occipital headaches vs M patients. Another important finding of our study is that we found a strong association between the ages of onset of headache and of vestibular symptoms in VM patients. This is consistent with the possibility that the 2 are mechanistically linked. A recent study independently found a similar result of association between headache onset and vestibular symptoms onset.18 The final finding is that the characteristics and symptomatology of patients suffering from VM and M do not completely overlap: (1) the headache location is more likely to be occipital in VM patients than in M patients (P < .001); (2) the age of onset of headache is higher in VM patients than in M patients (P < .001); and (3) VM seems to be associated with motion sickness more than M (P < .001). Therefore, a patient suffering from migraines with occipital location for their headaches, who developed migraine at an older age and who has motion sickness may be at a higher risk to develop VM down the line.
Comparing our results with prior literature, the onset of vestibular symptoms succeeds headache by several years, consistent with literature.6,11,12,19,20 Here we report an average age onset of vestibular symptoms of 32 years old; studies that specifically reported onset of vestibular symptoms in VM patients found a range from 7 to 7221 and 38.19 Other studies reported the average age of onset of VM and found that to be late 30 to 40 seconds or even later.2,6,9,22 Since vestibular symptoms generally occur later in life than headache symptoms, the age of onset of VM would correspond to the age of onset of vestibular symptoms in patients who develop migraine headaches first and later in life fulfill VM criteria. Concerning the co-occurrence of headache and vestibular symptoms, 46% of our patients reported vestibular symptoms and headaches associated together and 4% reported them independent of each other, consistent with another study reporting 48% and 5% respectively.23 However, Beh and colleagues reported that headache accompanied vestibular symptoms in half of their patients.3 It remains to be elucidated why VM headache and vestibular symptoms do not necessarily occur on the same day. We also found that VM patients are more likely to have personal and family histories of motion sickness than patients in the M group, in agreement with previous findings.23–25 The fact that motion sickness occurs in 43% of our VM sample is consistent with prior studies (42%–78%),3,19,23,25 although the motion sickness reported in our M group might have been underestimated (see limitations section).
VM Pathophysiology Supports an Occipital Location of the Headache.—
Recent studies with the goal of elucidating the pathophysiology of VM (VM specifically and as a separate entity from migraine) hint that structures located in the back of the head (posterior fossa) are involved. First, the vestibular system involves fibers that send signals from the vestibular ganglion to posterior fossa structures (the brain stem and cerebellum).26 Second, the involvement of the brainstem and cerebellum in migraine is already well documented,27–32 and finally in VM, the contribution of vestibular fibers within the brainstem and cerebellum is supported by the presence of documented central vestibular nystagmus during or between headaches in VM patients.33–36
Our study suggests the occipital location of headache is more common in VM than migraine. A recent study based on a questionnaire found that 23% of VM patients had “back of the head” location for their headaches, but this finding was not a primary outcome, was not further discussed, and there was no comparison to a migraine group without vestibular symptoms.19 A retrospective study from 1999 found that in patients with vertigo that could be related to migraine (this study predated VM criteria) found that 7/42 patients had “occipital” location for their headaches; but again, there was no comparison group.21
Interestingly, other pathologies that affect the posterior fossa can result in a headache in an occipital location.37 For example, half of patients with vertebral artery dissection suffer from vertigo/dizziness and headaches that are typically occipital.37–39 Patients with strokes in the posterior fossa, including lateral medullary infarcts, report vertigo/dizziness and occipital headaches.40 Patients with intracranial hemorrhages of the posterior fossa also have headaches located in the occipital area.41 Finally, Chiari malformation, an abnormality in the posterior fossa, causes vertigo/dizziness and occipital headaches.42 These various posterior fossa pathologies that cause vestibular symptoms and occipital headaches are consistent with our finding of the occipital location of headaches in VM patients.
Of note, it is important to be clear that VM is different from migraine with aura of vertigo (where vertigo happens prior to headaches and fulfills aura criteria according to ICHD3). In VM, the vestibular symptoms can happen before, during or after the headache or be independent of the headache.6
Limitations.—
A limitation of our study is the retrospective nature of the study which leaves gaps in the information for some patients. Another limitation is that the questionnaires used in the 2 clinics differed because 1 focused on the treatment of vertigo/dizziness/balance problems, whereas the other focused on the treatment of headaches. Two major differences in the surveys related to how the occipital pain symptomatology and motion sickness were identified. Regarding occipital pain, at the Balance Disorders Clinic, pain at the 2 sites, headache location or neck pain, was asked about in totally separate questions and the location question was open-ended, making it harder for the patients to answer in details, and likely biasing the result for this group toward underestimating a possible occipital location of the headache. On the other hand, patients at the Headache Clinic were asked to check boxes corresponding to the location of their headache, and occipital/back of the head and neck pain were all lumped together. Knowing that neck pain is very common in the general population, including in M and VM patients,43 therefore, if we were able to remove all neck pain cases from the M group, the presence of occipital headache would possibly have been even less pronounced in the M group. Therefore, the difference in the questionnaires possibly led to an underestimation of the actual VM and M difference.
The second important difference in the questionnaires relates to how motion sickness was reported. At the Balance Disorders Clinic, patients were asked specifically if they had motion sickness, by asking a yes/no question whether they had car sickness. At the Headache Clinic, the question was not asked directly, but some patients penciled in motion sickness or car sickness under “other” symptoms. We suspect that this difference accounted for the fact that far less motion sickness was reported in the M group than is expected from prior literature.44
Another limitation is that our sample does not reflect the estimated proportion of VM to M in the general population. Even though VM is underreported, and thus the exact VM to M proportion is not known, we still suspect that the VM to M ratio in our simple random sample does not reflect the general population. A prospective study can confirm these preliminary findings. A final limitation of this study is that it does not include a population of migraine patients who suffer from vertigo/dizziness yet do not fulfill the diagnostic criteria for VM. A prospective study is needed to include those patients, as well as comparing the patients with different vestibular symptomatology within the VM group and those with and without aura. Nevertheless, this questionnaire-based study provides information supporting the notion that there are key differences in the location of headache in VM vs migraine patients, and in prior VM studies, questionnaires have shown good validity and reliability.4,19
CONCLUSIONS
Although vestibular migraine shares numerous features with migraine,11 we found that VM patients’ symptomatology does not completely overlap. The headaches in VM patients are more likely to be occipital than those of migraine patients. VM patients develop headaches later in life and are more likely to have motion sickness than M patients. Although the number of clinical studies of VM is increasing, the number that directly compares the symptomatology of VM patients to that of migraineurs is still too low to be able to draw strong conclusions about the meaning of these observations. Future prospective longitudinal studies will allow better discrimination between VM and M conditions, and possibly lead to the identification of distinct treatments for more effective alleviation of the pain, and to increases in the quality of life for many patients. The results of this retrospective study support the initiation of a prospective study investigating whether a patient who develops migraine headache later in life, has pain localized in the back of the head, and has a history of motion sickness is more likely to suffer from vestibular migraine at a later age.
Acknowledgments:
The authors thank Anne Luebke for helpful comments on data interpretation.
Funding: This work was supported by a Merit Review Award A-ORD 1IO1RX002101, a Career Development Award IK2 RX002010 012020, a Pilot Project awarded under The Center for the Prevention and Treatment of Visual Loss Award Number C9251-C from the United States (U.S.) Department of Veterans Affairs, Rehabilitation Research and Development Service, NIH R01 NS075599, and NIH R01 DC017261. The contents of this paper do not represent the views of the VA or the United States Government.
Abbreviations:
- CI
confidence interval
- M
migraine only
- OR
odds ratio
- VM
vestibular migraine
Footnotes
Conflict of Interest: None
Trial Registration: University of Iowa Institutional IRB (#201404723).
REFERENCES
- 1.Dieterich M, Obermann M, Celebisoy N. Vestibular migraine: The most frequent entity of episodic vertigo. J Neurol. 2016;263(Suppl. 1):S82–S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beh SC. Vestibular migraine: How to sort it out and what to do about it. J Neuroophthalmol. 2019; 39:208–219. [DOI] [PubMed] [Google Scholar]
- 3.Beh SC, Masrour S, Smith SV, Friedman DI. The spectrum of vestibular migraine: Clinical features, triggers, and examination findings. Headache. 2019;59:727–740. [DOI] [PubMed] [Google Scholar]
- 4.Celebisoy N, Karapolat H, Gokcay F, et al. Establishing a “vestibular migraine diagnosis questionnaire” and testing its validity. Neurologist. 2016;21:51–54. [DOI] [PubMed] [Google Scholar]
- 5.Geser R, Straumann D. Referral and final diagnoses of patients assessed in an academic vertigo center. Front Neurol. 2012;3:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang TC, Wang SJ, Kheradmand A. Vestibular migraine: An update on current understanding and future directions. Cephalalgia. 2020;40:107–121. [DOI] [PubMed] [Google Scholar]
- 7.Millen SJ, Schnurr CM, Schnurr BB. Vestibular migraine: Perspectives of otology versus neurology. Otol Neurotol. 2011;32:330–337. [DOI] [PubMed] [Google Scholar]
- 8.Neuhauser H, Leopold M, von Brevern M, Arnold G, Lempert T. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology. 2001;56:436–441. [DOI] [PubMed] [Google Scholar]
- 9.Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: Prevalence and impact on quality of life. Neurology. 2006;67:1028–1033. [DOI] [PubMed] [Google Scholar]
- 10.Sohn JH. Recent advances in the understanding of vestibular migraine. Behav Neurol. 2016;2016:1801845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baloh RW. Vestibular migraine I: Mechanisms, diagnosis, and clinical features. Semin Neurol. 2020;40:76–82. [DOI] [PubMed] [Google Scholar]
- 12.Lempert T, Olesen J, Furman J, et al. Vestibular migraine: Diagnostic criteria. J Vestib Res. 2012; 22:167–172. [DOI] [PubMed] [Google Scholar]
- 13.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 14.Formeister EJ, Rizk HG, Kohn MA, Sharon JD. The epidemiology of vestibular migraine: A population-based survey study. Otol Neurotol. 2018;39:1037–1044. [DOI] [PubMed] [Google Scholar]
- 15.Cho SJ, Kim BK, Kim BS, et al. Vestibular migraine in multicenter neurology clinics according to the appendix criteria in the third beta edition of the International Classification of Headache Disorders. Cephalalgia. 2016;36:454–462. [DOI] [PubMed] [Google Scholar]
- 16.Shin JH, Kim YK, Kim HJ, Kim JS. Altered brain metabolism in vestibular migraine: Comparison of interictal and ictal findings. Cephalalgia. 2014;34:58–67. [DOI] [PubMed] [Google Scholar]
- 17.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- 18.Teggi R, Colombo B, Albera R, et al. Clinical features, familial history, and migraine precursors in patients with definite vestibular migraine: The VM-phenotypes projects. Headache. 2018;58:534–544. [DOI] [PubMed] [Google Scholar]
- 19.Teggi R, Colombo B, Albera R, et al. Clinical features of headache in patients with diagnosis of definite vestibular migraine: The VM-phenotypes projects. Front Neurol. 2018;9:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Brevern M, Lempert T. Vestibular migraine. Handb Clin Neurol. 2016;137:301–316. [DOI] [PubMed] [Google Scholar]
- 21.Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): Vestibular migraine? J Neurol. 1999;246:883–892. [DOI] [PubMed] [Google Scholar]
- 22.Power L, Shute W, McOwan B, Murray K, Szmulewicz D. Clinical characteristics and treatment choice in vestibular migraine. J Clin Neurosci. 2018;52:50–53. [DOI] [PubMed] [Google Scholar]
- 23.Boldingh MI, Ljostad U, Mygland A, Monstad P. Vestibular sensitivity in vestibular migraine: VEMPs and motion sickness susceptibility. Cephalalgia. 2011;31:1211–1219. [DOI] [PubMed] [Google Scholar]
- 24.Furman JM, Marcus DA. Migraine and motion sensitivity. Continuum (Minneap Minn). 2012;18: 1102–1117. [DOI] [PubMed] [Google Scholar]
- 25.Jeong SH, Oh SY, Kim HJ, Koo JW, Kim JS. Vestibular dysfunction in migraine: Effects of associated vertigo and motion sickness. J Neurol. 2010;257:905–912. [DOI] [PubMed] [Google Scholar]
- 26.Balmer TS, Trussell LO. Selective targeting of uni-polar brush cell subtypes by cerebellar mossy fibers. eLife. 2019;8:e44964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahra A, Matharu MS, Buchel C, Frackowiak RS, Goadsby PJ. Brainstem activation specific to migraine headache. Lancet. 2001;357:1016–1017. [DOI] [PubMed] [Google Scholar]
- 28.Chong CD, Plasencia JD, Frakes DH, Schwedt TJ. Structural alterations of the brainstem in migraine. Neuroimage Clin. 2017;13:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrusic I, Dakovic M, Zidverc-Trajkovic J. Volume alterations of brainstem subregions in migraine with aura. Neuroimage Clin. 2019;22:101714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harno H, Hirvonen T, Kaunisto MA, et al. Subclinical vestibulocerebellar dysfunction in migraine with and without aura. Neurology. 2003;61:1748–1752. [DOI] [PubMed] [Google Scholar]
- 31.Noseda R, Melo-Carrillo A, Nir RR, Strassman AM, Burstein R. Non-trigeminal nociceptive innervation of the posterior dura: Implications to occipital headache. J Neurosci. 2019;39:1867–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerwig M, Rauschen L, Gaul C, Katsarava Z, Timmann D. Subclinical cerebellar dysfunction in patients with migraine: Evidence from eyeblink conditioning. Cephalalgia. 2014;34:904–913. [DOI] [PubMed] [Google Scholar]
- 33.Bednarczuk NF, Bonsu A, Ortega MC, et al. Abnormal visuo-vestibular interactions in vestibular migraine: A cross sectional study. Brain. 2019;142:606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polensek SH, Tusa RJ. Nystagmus during attacks of vestibular migraine: An aid in diagnosis. Audiol Neurootol. 2010;15:241–246. [DOI] [PubMed] [Google Scholar]
- 35.Vincent M, Hadjikhani N. The cerebellum and migraine. Headache. 2007;47:820–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Brevern M, Zeise D, Neuhauser H, Clarke AH, Lempert T. Acute migrainous vertigo: Clinical and oculographic findings. Brain. 2005;128:365–374. [DOI] [PubMed] [Google Scholar]
- 37.Moskowitz MA, Buzzi MG, Sakas DE, Linnik MD. Pain mechanisms underlying vascular headaches. Progress Report 1989. Rev Neurol (Paris). 1989;145:181–193. [PubMed] [Google Scholar]
- 38.de Sousa JE, Halfon MJ, Bonardo P, Reisin RC, Fernandez Pardal MM. Different pain patterns in patients with vertebral artery dissections. Neurology. 2005;64:925–926. [DOI] [PubMed] [Google Scholar]
- 39.Gottesman RF, Sharma P, Robinson KA, et al. Clinical characteristics of symptomatic vertebral artery dissection: A systematic review. Neurologist. 2012;18:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JS. Pure lateral medullary infarction: Clinical-radiological correlation of 130 acute, consecutive patients. Brain. 2003;126:1864–1872. [DOI] [PubMed] [Google Scholar]
- 41.Melo TP, Pinto AN, Ferro JM. Headache in intracerebral hematomas. Neurology. 1996;47:494–500. [DOI] [PubMed] [Google Scholar]
- 42.Panconesi A, Macucci M, Bartolozzi ML, Mugnai S, Guidi L. Investigation on occipital headache associated with vertigo and vomiting discovers a familial clustering of Chiari I malformation and a “puzzle”. J Headache Pain. 2015;P021:A172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lampl C, Rudolph M, Deligianni CI, Mitsikostas DD. Neck pain in episodic migraine: Premonitory symptom or part of the attack? J Headache Pain. 2015;16:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marcus DA, Furman JM, Balaban CD. Motion sickness in migraine sufferers. Expert Opin Pharmacother. 2005;6:2691–2697. [DOI] [PubMed] [Google Scholar]


