Abstract
Systemic glucocorticoids remain the standard treatment for gastrointestinal (GI) acute graft-versus-host disease (aGVHD) despite their toxicity and incomplete efficacy. Controlled trials have tested poorly absorbable steroids as adjuncts with systemic glucocorticoids, but only small case series have reported treatment with poorly absorbed beclomethasone dipropionate (BDP) and budesonide (BUD) alone. Our team has adopted the practice of administering BDP or BDP+BUD without systemic glucocorticoids as first-line therapy for isolated upper GI (UGI) aGVHD. We report results in 76 patients treated with BDP alone and in 81 patients treated with BDP+BUD, with allocation by physician choice. Almost all patients received peripheral blood stem cells (92%) from a fully HLA-matched related or unrelated donor (80%) after myeloablative conditioning (76%) for acute leukemia (49%), myelodysplastic syndrome (17%), non-Hodgkin lymphoma (14%), or another hematopoietic disorders (20%). After 28 days of treatment with BDP, 46% of the patients had a complete response (CR) and 10% had a partial response (PR); after 200 days, 61 (80%) patients were alive, 34% maintained a CR, and 3% maintained a PR, whereas 53% required additional immunosuppression (IS). After 28 days of treatment with BDP+BUD, 67% had a CR and 10% a PR; after 200 days, 74 (91%) patients were alive, 46% maintained a CR, and 2% maintained a PR, whereas 43% required additional IS. Among the entire cohort of 157 patients, 66 (42%) were treated successfully without systemic glucocorticoids. This study reports the efficacy of poorly absorbable steroids alone for patients with isolated UGI aGVHD. Prospective trials should test for the potential advantages of BDP and BUD use over systemic glucocorticoids.
Keywords: Upper gastrointestinal acute, GVHD, Beclomethasone, Budesonide, Hematopoietic stem cell transplantation
INTRODUCTION
Gastrointestinal (GI) acute graft-versus-host disease (aGVHD) is a common and severe complication of allogeneic hematopoietic stem cell transplantation (HCT) [1]. Upper GI (UGI) aGVHD, classified as stage 1 GI GVHD and overall grade II aGVHD, is reported approximately in 30% of HCT recipients with GVHD and is characterized by anorexia, food intolerance, nausea, and vomiting without abdominal cramps or diarrhea; in almost one-quarter of patients, UGI symptoms are present in isolation [2–4]. Isolated UGI aGVHD appears to be more responsive to immunosuppressive therapy than aGVHD involving other areas of the gut. Systemic glucocorticoids remain the standard of care [5] despite their incomplete efficacy and toxicity, as well as the risk of hampering the antileukemic effect of the graft. The outcomes of various GI aGVHD treatments remain far from satisfactory, with a high rate of poor response to immunosuppressive treatments and a high mortality rate [6–8]. Novel systemic steroid-sparing strategies have been tested in attempts to improve the management of GI aGVHD, and some of them seem promising [9–11].
For more than a decade, the nonabsorbable corticosteroids beclomethasone dipropionate (BDP) [12] and budesonide (BUD) [13] have been used to treat mild to moderate GI GVHD, based on their encouraging results in inflammatory bowel diseases [14–17]. Two randomized trials showed systemic steroid-sparing activity and improved survival in patients with UGI aGVHD treated with a combination of BDP plus systemic glucocorticoids compared with those treated with systemic glucocorticoids alone [18,19]. Despite these encouraging results and the low toxicity profile, in 2007 the US Food and Drug Administration (FDA) Oncology Drugs Advisory Committee considered the evidence from a registration trial of one formulation of BDP to be insufficient and voted against approving BDP for the treatment of GI GVHD, precluding its meaningful adoption in everyday clinical practice. The use of institutionally formulated BDP continues because of the lack of other safe and effective GI GVHD treatments, despite the paucity of supporting clinical data in HCT [20]. BUD, used in aGVHD along with systemic corticosteroids, has shown good tolerability, a high response rate, and systemic steroid-sparing activity and is now included in the European guidelines as a first-line option for GI aGVHD [21,22]. Even more limited data in small patient series support the use of BDP or BDP+BUD without concomitant systemic glucocorticoids. BDP as single-agent therapy has been tested in phase I [23] and phase II [24,25] studies and after cord blood transplantation [26,27], whereas the efficacy of sole BUD has been examined only in patients with GI chronic GVHD (cGVHD) [28].
Our team has adopted the practice of administering BDP alone or BDP+BUD without systemic glucocorticoids as a first-line strategy to treat UGI aGVHD. To the best of our knowledge, there are no data on the role of BDP alone or BDP+BUD in this setting. Patients with UGI disease often progress to symptomatic lower GI (LGI) involvement [20,29–31]. Whereas BDP reaches the esophagus and stomach but not beyond the duodenum, BUD is released from capsules in the stomach, percolates the ileum and reaches the colon, where it is partly reabsorbed. Therefore, the combination of BDP+BUD was instituted to prevent LGI GVHD involvement. The therapeutic effect of BUD is local, because its systemic bioavailability is extremely low due to a rapid first-pass metabolism in the liver [21–27].
The aim of the present study was to retrospectively analyze the response after 28 days of treatment with poorly absorbable glucocorticoids, BDP as single agent or in combination with BUD, in a cohort of isolated UGI aGVHD patients. Secondary goals included response at day 200, treatment failure and mortality.
METHODS
Study Design
Clinical and laboratory data of 157 consecutive adult patients age >18 years with isolated UGI aGVHD [3] after HCT performed at Moffitt Cancer Center between July 2004 and June 2013 were retrospectively collected. The study was approved by the Moffitt Cancer Center’s Scientific Review Committee and Institutional Review Board. This research posed no risks to the human subjects involved and was conducted in accordance with the Declaration of Helsinki and the guidelines for Good Clinical Practice.
Study Population
Only patients with symptoms of isolated UGI aGVHD according to Przepiorka et al [3] (eg, anorexia, food intolerance, nausea, vomiting) were included in this study. When feasible, the diagnosis of GVHD was confirmed with endoscopic biopsies of the UGI plus LGI tract, following standard protocol according to Spencer et al [32]. Patients with concomitant aGVHD of skin, stage ≤1 without a requirement for systemic treatment except topical agents at the time of UGI aGVHD onset were included in this study. Conversely, patients with any stage of LGI aGVHD, aGVHD skin stage >1, or any stage of liver aGVHD at the time of UGI aGVHD onset were excluded. A documented bacterial, fungal, or viral enteric infection was another exclusion criterion. Patients receiving corticosteroids for indications other than GVHD within 30 days of study entry were also excluded. We identified 297 recipients of HCT performed between July 2004 and June 2013 with aGVHD involving the UGI and/or LGI tract, including those with stage ≤1 skin aGVHD and without any stage of liver aGVHD. Based on our study’s inclusion and exclusion criteria, 157 of them (53%) had features compatible with isolated UGI aGVHD and composed the study population.
Treatment Procedures
Patients who met the inclusion criteria were treated, based on the clinical decision of the attending physician, with the topical steroids BDP and BUD, according to 1 of 2 treatment regimens: BDP alone or BDP+BUD. BDP was administered orally as a 5 mg/5 mL liquid oil formulation twice daily, and BUD was administered orally as a 3 mg tablet twice daily. Tapering of BDP and BUD after aGVHD response was directed by the treating physician.
Study Endpoints
The primary study endpoint was UGI aGVHD response after 28 days of treatment. Response was defined as complete response (CR) in cases of resolution of all UGI symptoms without addition of other immunosuppressive (IS) agents or as partial response (PR) in cases of improved UGI symptoms from baseline without resolution, without worsening, and without the addition of other IS agents [19,24,25,27]. Patients were classified as nonresponders in the event of local treatment failure (ie, no improvement, worsening, or recurrence of UGI aGVHD symptoms requiring additional IS), systemic treatment failure (ie, requirement of systemic IS for GVHD other than GI), or local plus systemic treatment failure. Patients were considered not evaluable in the event of malignancy relapse or death before study evaluation time points. Time to additional IS agents was defined as the interval from UGI aGVHD onset to the date of additional IS agent administration; death was considered a competing risk for additional IS agents. Additional IS agents included systemic prednisone, sirolimus, mycophenolate mofetil, infliximab, rituximab, and photopheresis, used as single-agent or combination therapy.
Secondary study endpoints were UGI aGVHD response after 200 days of treatment, overall survival (OS), cause of death, cytomegalovirus (CMV) reactivation, and cumulative incidence of cGVHD according to Filipovich et al [33]. CMV reactivation was defined as CMV serum positivity (>1000 CMV DNA copies/mL of blood for an HLA-matched unrelated donor and an HLA-matched related donor or >250 CMV copies/mL for cord blood), CMV disease (by rapid cultures, direct fluorescent antibody tests, DNA hybridization, or cytology), or CMV PCR positivity in bronchoalveolar lavage, GI tract, or cerebrospinal fluid. Relapse and nonrelapse mortality were considered competing risks for cGVHD development.
Statistical Analysis
Baseline characteristics and endpoints were analyzed by treatment arm, and Pvalues were computed using the Fisher exact test or Wilcoxon rank-sum test. The association between treatment arm and response at days +28 and +200 was evaluated using simple and multiple logistic regression models. The association of treatment arm with OS was estimated using univariate and multivariate Cox proportional hazard models. Variables were entered into the multivariate model based on a univariate regression P value < .25 and were kept in the final model if the P value was < .1 during backward selection; variables with P> .1 were eliminated. The associations between treatment arm and endpoints were adjusted for factors affecting aGVHD response or death, including age, diagnosis, conditioning regimen, disease status before HCT, donor and recipient characteristics, treatment arm, albumin level at baseline, GI biopsy results, CMV serostatus, and time of onset of UGI aGVHD after HCT.
Survival was analyzed using the Kaplan-Meier method, and curves were compared using the log-rank test. Pointwise 95% confidence intervals (CIs) for survival curves were computed using log-log transformation. Conventional methods were used for statistical analysis (JMP 14.0.0; SAS Institute, Cary, NC).
RESULTS
Among all 157 patients with isolated UGI aGVHD, 76 were treated with BDP alone and 81 were treated with BDP+BUD. Almost all patients received peripheral blood stem cells (92%) from a fully HLA-matched donor (80%) after conditioning with a myeloablative regimen (76%) for acute leukemia (49%), myelodysplastic syndrome (17%), or another hematopoietic disorder. aGVHD prophylaxis was tacrolimus/methotrexate in 52%, tacrolimus/sirolimus in 30%, tacrolimus/mycophenolate mofetil in 12%, and cyclosporine/mycophenolate mofetil in 6%. Almost two-thirds (63%) of the patients had UGI aGVHD confirmed by GI biopsy of the upper and lower tract.
Baseline Characteristics of the Study Population
Baseline characteristics of the study cohort are presented in Table 1. Treatment arms were well balanced in terms of demographic variables, primary disease, risk category at HCT, stem cell source, aGVHD prophylaxis, and CMV status. A borderline significant difference between the treatment groups was noted for donor type and time from UGI aGVHD onset. A matched related donor was used in 26 recipients (34%) treated with BDP and in 37 recipients (46%) treated with BDP+BUD (P= .04). The onset of UGI aGVHD was later in recipients treated with BDP alone compared with those treated with BDP+BUD (median, 31 days versus 26 days post-HCT; P= .03). Furthermore, the conditioning regimen was more often myeloablative in the BDP+BUD group compared with the BDP group (88% versus 63%; P= .0003) and GI biopsy was performed more frequently in the BDP+BUD group (74% versus 51%; P= .0003).
Table 1.
Baseline Characteristics of 157 Patients Grouped by BDP or BDP+BUD Treatment for UGI aGVHD
| Variable | BDP(n = 76) | BDP+BUD (n = 81) | P Value |
|---|---|---|---|
| Recipient age, yr, median (range) | 52 (22–76) | 54 (23–70) | .8 |
| Recipient/donor sex, n (%) | .8 | ||
| Male/male | 27(35) | 35 (43) | |
| Female/female | 16(21) | 14(17) | |
| Female/male | 22 (29) | 20(25) | |
| Male/female | 11(15) | 12(15) | |
| Diagnosis, n (%) | .2 | ||
| Acute leukemia | 35 (46) | 42(52) | |
| MDS | 14(18) | 12(15) | |
| B/T-NHL, HD, CLL | 8(11) | 15(18) | |
| Other* | 19(25) | 12(15) | |
| CIBMTR risk category at HCT, n (%)† | .2 | ||
| Low | 29 (39) | 36(45) | |
| Intermediate | 17(23) | 20(25) | |
| High | 27(38) | 21(26) | |
| Nonmalignant/stable disease | 0 | 3(4) | |
| Conditioning regimen, n (%) | .0003 | ||
| Myeloablative | 48 (63) | 71 (88) | |
| Reduced intensity | 28(37) | 10(12) | |
| Stem cell source, n (%) | .07 | ||
| Peripheral blood | 66 (86) | 78 (96) | |
| Bone marrow | 1(1) | 1(1) | |
| Cord blood, double | 9(13) | 2(3) | |
| Donor type, n (%) | .04 | ||
| MRD | 26 (34) | 37(46) | |
| MUD | 41 (54) | 42(53) | |
| Cord blood | 9(12) | 2(3) | |
| Acute GVHD prophylaxis, n (%) | .2 | ||
| CSA/MMF | 8(10) | 2(3) | |
| TAC/MMF | 9(12) | 10(13) | |
| TAC/MTX | 35 (46) | 46 (57) | |
| TAC/SIRO | 24(32) | 23 (27) | |
| ATG or rituximab treatment, n (%)‡ | .6 | ||
| ATG | 9(12) | 8(10) | |
| Rituximab | 3(4) | 5(6) | |
| ATG+rituximab | 1(1) | 0 | |
| Neither ATG nor rituximab | 63 (83) | 68 (84) | |
| Donor/recipient CMV status, n (%) | .9 | ||
| Negative/negative | 17(22) | 18(22) | |
| Negative/positive | 24(32) | 26(32) | |
| Positive/negative | 8(10) | 11(14) | |
| Positive/positive | 25(33) | 25(31) | |
| Other§ | 2(3) | 1(1) | |
| GI biopsy, n (%)‖ | 39(51) | 60 (74) | .003 |
| Day of UGI aGVHD onset, median (range) | 31 (14–174) | 26(10–253) | .03 |
| Acute skin stage 1 GVHD, n (%) | 25(33) | 30 (37) | .6 |
Significant P values (< .05) are in bold type.
MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; CIBMTR, Center for International Blood and Marrow Transplant Research; MRD, matched related donor; MUD, matched unrelated donor; CSA, cyclosporine; MMF, mycophenolate mofetil; TAC, tacrolimus/mycophenolate mofetil; MTX, methotrexate; SIRO, sirolimus; ATG, antithymocyte globulin.
Includes aplastic anemia, myeloproliferative disorders including chronic myelogenous leukemia, paroxysmal nocturnal hemoglobinuria, and multiple myeloma.
Percentages do not always add up to 100, because of rounding and missing data, but refer to the total number of scored events (n evaluable = 153).
Too few patients that received rituximab and/or ATG (<20 in each group) to allow for a meaningful stratified analysis of response to BDP versus BDP+BUD.
Not available.
When feasible, endoscopy with biopsy of the UGI and LGI tract was performed (stomach plus duodenum plus rectum), resulting in a high rate of histologically proven aGVHD: 99 (63%) patients underwent an endoscopy with biopsy. We recorded 11 patients (11%) positive for only UGI, 79 (80%) positive for both UGI and LGI without documented diarrhea, 5 (5%) positive for only LGI without documented diarrhea, and 4 (4%) histologically equivocal. Globally, 58 patients (37%) patients with clinical symptoms of isolated UGI aGVHD and without a diagnostic biopsy of the GI tract were included in the study based on the physician decision to treat UGI aGVHD. These patients were negative for bacterial, fungal, or viral enteric infection and for other exclusion criteria: skin aGVHD stage <1 treated systemically, skin aGVHD stage >1, any stage of LGI aGVHD, any stage of liver aGVHD, or treatment with corticosteroids for indications other than GVHD within 30 days of study entry.
Treatment Characteristics
Based on physician choice, patients were treated with BDP alone (n = 76) or BDP+BUD (n = 81). BDP was administered orally as a 5 mg/5 mL liquid oil formulation twice daily in all but 3 patients treated with BDP 3 times daily, whereas BUD was given orally as a 3 mg tablet twice daily in all but 1 patient treated with BUD once daily. The duration of treatment was longer in the BDP+BUD group (median, 225 days [range, 10 to 1176 days] versus 96 days [range, 7 to 816 days] with BDP; P< .0001) (Table 2).
Table 2.
Response to BDP or BDP+BUD Treatment for UGI aGVHD at Days +28 and +200 after Treatment Start
| Variable | BDP(n = 76) | BDP+BUD (n = 81) | P Value |
|---|---|---|---|
| Response at day 28, n (%) | .2 | ||
| CR | 40 (53) | 54(67) | |
| PR | 8(10) | 8(10) | |
| Failure | 28 (37) | 18(22) | |
| Not evaluable* | 0 | 1(1) | |
| Response at day 200, n (%) | .2 | ||
| CR | 26 (34) | 37 (46) | |
| PR | 2(3) | 2(2) | |
| Failure | 31(41) | 33 (41) | |
| Not evaluable† | 17(22) | 9(11) | |
| Treatment duration, d, median (range)‡ | 96(7–816) | 225(10–1176) | <.0001 |
Significant P values (<.05) are in bold type.
Data missing.
22 were dead at day 200, and 4 were lost to follow-up.
Patients with treatment duration <28 days (n = 6 patients in the BDP group and 8 in the BDP+BUD group) experienced CR, treatment failure with requirement of other additional IS agent, or death before the day 28 evaluation. All patients who achieved CR with short-course therapy maintained the CR through the day 200 evaluation.
Response to Treatment
Response to treatment is summarized in Table 2 and in Figure 1. After 28 days of treatment, 40 patients (53%) treated with BDP were in CR and 8 (10%) were in PR, whereas 54 (67%) patients treated with BDP+BUD were in CR and 8 (10%) were in PR. Twenty-eight patients (37%) did not respond to treatment with BDP, and 18 (22%) did not respond to treatment with BDP+BUD. In univariate analysis, a slightly better response was seen with BDP+BUD compared with BDP alone (odds ratio [OR], 2; 95% CI, 1 to 4; P= .049). However, after adjusting for patient age, recipient/donor sex, diagnosis, conditioning regimen intensity, CMV serostatus, aGVHD onset after HCT, albumin level at baseline, donor type, disease status before HCT, aGVHD prophylaxis, disease status at HCT, GI biopsy findings, and donor relation, treatment with BDP+BUD was not associated with a better response after 28 days on multivariate analysis (Supplementary Table S1).
Figure 1.
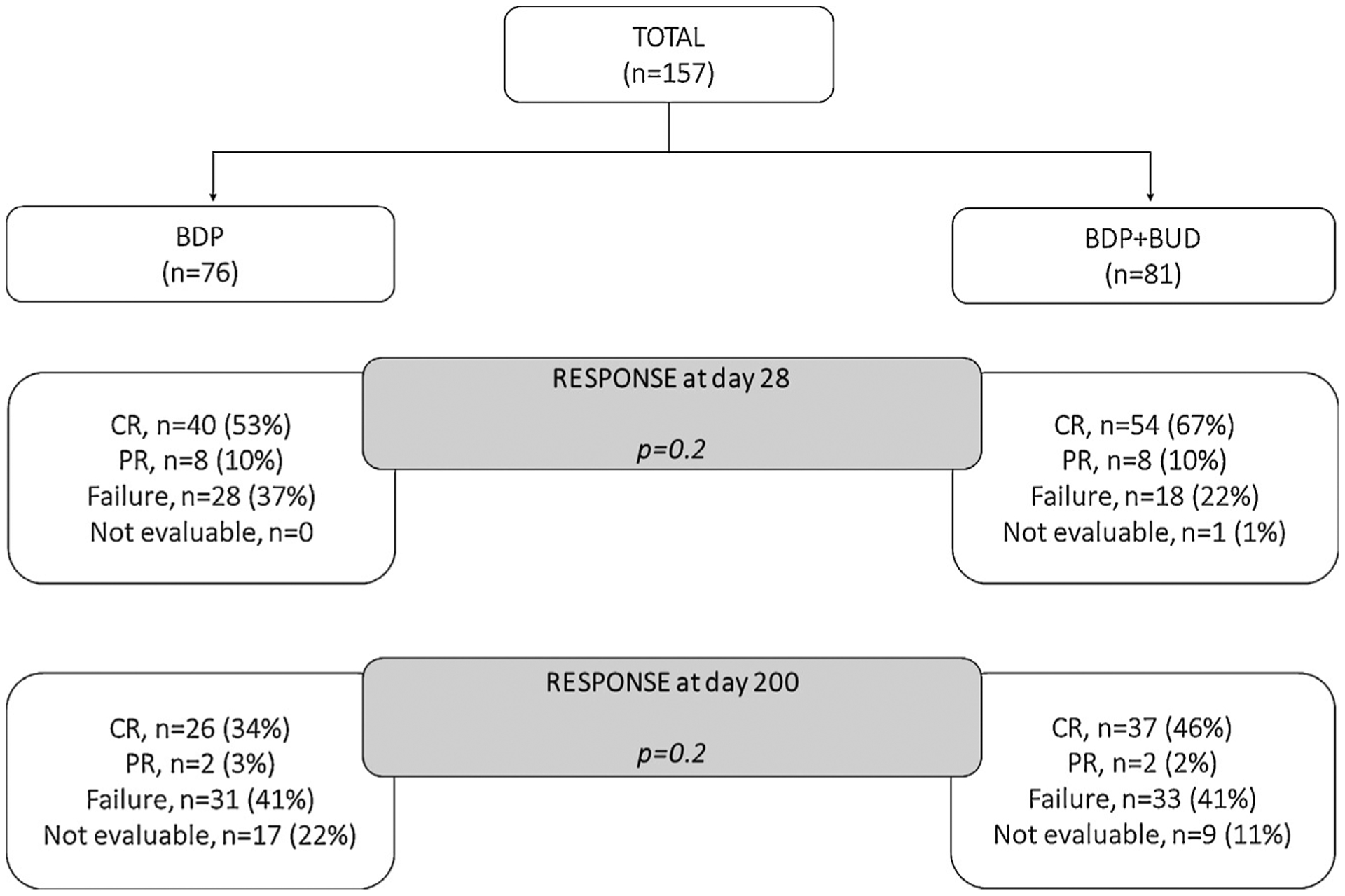
CONSORT diagram with response and failure of the study population.
After 200 days, responses remained similar in the 2 treatment groups (P= .2) (Table 2). At this time point, 61 patients (80%) were alive in the BDP arm, compared with 74 (91%) in the BDP+BUD arm. After treatment with BDP alone, 26 patients remained in CR (34%), 2 were in PR (3%), 31 failed therapy (41%) and 17 (22%) were not evaluable due to death, malignancy relapse, or missing data before the study evaluation time point. After treatment with BDP+BUD, 37 patients were in CR (46%), 2 were in PR (2%), 33 (41%) failed the first-line treatment for UGI aGVHD, and 9 (11%) were not evaluable. In multivariate analysis, complete responses were not significantly different between the 2 groups (Supplementary Table S1).
The most common regimens for aGVHD prophylaxis in the entire cohort were methotrexate in combination with tacrolimus, administered to 81 patients (52%), and sirolimus in combination with tacrolimus, administered to 47 patients (30%) patients (Table 1). The proportions of patients treated with BDP or with BDP+BUD were similar in patients administered methotrexate or sirolimus for GVHD prophylaxis; among those administered methotrexate, 46% were treated with BDP and 57% were treated with BDP+BUD, whereas among those given sirolimus, 32% received BDP and 27% received BDP+BUD (P= .2). There was no difference in OR at day 28 by GVHD treatment with BDP or BDP+BUD after stratifying by GVHD prophylaxis with methotrexate (OR, 46% with BDP versus 57% with BDP+BUD) or sirolimus (OR, 83% with BDP versus 78% with BDP+BUD) (P= .09). However, OR at day 28 after GVHD treatment with either BDP or BDP+BUD was higher among patients who had received sirolimus rather than methotrexate for GVHD prophylaxis (OR, 81% versus 64%; P= .04).
Sparing of Systemic Steroid Treatment
Among all 157 patients, 66 (42%) were successfully treated with sole nonabsorbable corticosteroid therapy for their UGI aGVHD, achieving CR or PR without the need for additional IS agents after 200 days of treatment. This group included 27 patients (36%) with BDP and 39 (48%) with BDP+BUD (P= .7).
Additional IS Agents
Among the entire study population treated with either BDP or BDP+BUD, we observed GVHD progression in 46 patients (22%) after 28 days from the start of treatment and in 64 patients (41%) after 200 days from the start of treatment. Progression of GVHD was largely localized to the GI tract: after 28 days, we recorded 41 (89%) local treatment failures, defined as no improvement in, worsening of, or recurrence of UGI aGVHD symptoms necessitating additional immunosuppression, and 5 (11%) systemic treatment failures, defined as GVHD progression in organs other than GI necessitating additional immunosuppression. After 200 days, we observed 49 (76%) local treatment failures, 10 (16%) systemic treatment failures, and 5 (8%) local plus systemic treatment failures.
Response to second-line aGVHD therapy with systemic glucocorticoids (n = 46) or other IS agents (n = 16) after BDP or BDP+BUD failure was assessed in 62 evaluable patients. By 28 days after second-line therapy, 37 patients (58%) achieved CR and 22 (34%) achieved PR; thus, 95% of evaluable patients achieved OR, whereas 5% did not improve. By day 200 after initial therapy with BDP or BDP+BUD and failure to respond, 41 patients (67% of 61 evaluable patients) achieved CR, 7 (11%) achieved PR, 5 (8%) did not improve, and 8 (13%) developed cGVHD. Therefore, failure to respond to initial therapy with poorly absorbable glucocorticoids did not appear to compromise subsequent GVHD control by standard therapy.
The proportion of patients requiring 1 or more IS agent(s) due to aGVHD worsening or progression within 200 days from the start of UGI aGVHD treatment did not differ between the 2 treatment groups, BDP versus BDP+BUD. We recorded additional IS agents in 40 patients (53%) initially treated with BDP and in 35 (43%) initially treated with BDP+BUD (P= .2) (Figure 2A). Systemic prednisone was added for 34 patients (42%) initially treated with BDP+BUD and for 23 (30%) initially treated with BDP (P< .0001). Conversely, an IS agent other than systemic prednisone was added for 1 patient (1%) initially treated with BDP+BUD and for 17 patients (22%) initially treated with BDP. There was a suggestion of an earlier requirement for an additional IS agent after treatment with BDP compared with BDP+BUD; the median time to additional IS agent (s) was 14 days (range, 3 to 176 days) after BDP, compared with 29 days (range, 1 to 176 days) after BDP+BUD (P= .07) (Figure 2B). The rate of early death among patients requiring an additional IS agent was similar in the 2 arms: 16 (40%) after BDP versus 13 (37%) after BDP+BUD.
Figure 2.
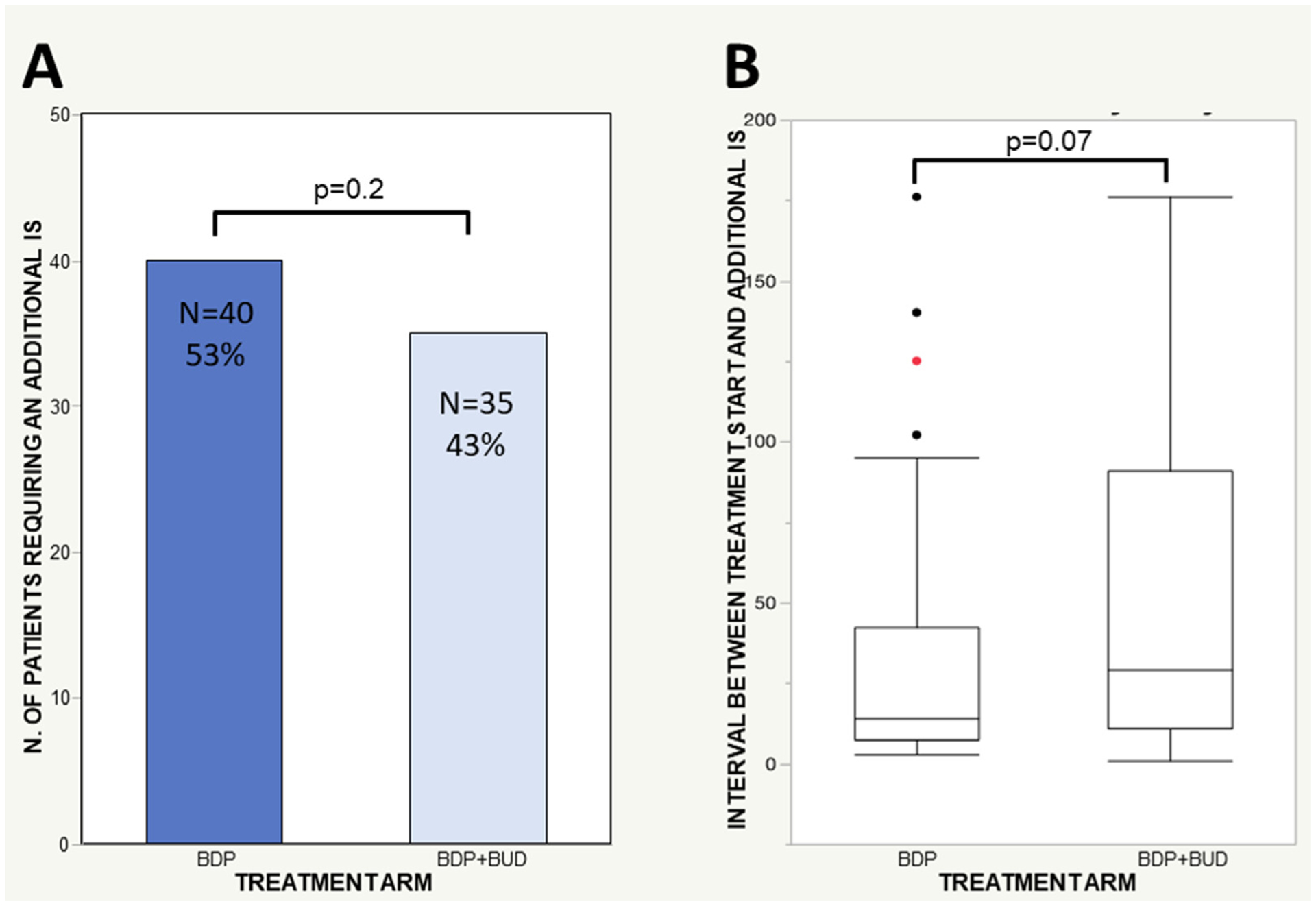
Additional IS agents. (A) Percentage of treatment failures at day 200 by study arm (BDP versus BDP+BUD) and proportion of patients requiring an additional IS agent(s) by study arm. (B) Time to treatment failure by treatment arm. Shown is the interval between the start of treatment for UGI aGVHD and the addition of another IS agent due to first-line treatment failure. The number of patients requiring an additional IS agent included all patients who required an additional IS agent by day 200 (n = 40 in the BDP cohort and n = 35 in the BDP+BUD cohort). These numbers are not equal to the number of failures at day 200, because failures included patients with aGVHD treatment failure requiring an additional IS agent (local treatment failure, systemic treatment failure, or local and systemic treatment failure) plus patients not evaluable at that time point owing to death or hematologic relapse before time point evaluation. For example, some patients required an additional IS by day 28 but then died or had a hematologic relapse before the day 200 evaluation and thus were classified as “not evaluable” at the day 200 response evaluation. In the BDP group: n = 40 additional IS (at day 28, n = 28 failures; at day 200, n = 31 failures, n = 9 not evaluable); in the BDP+BUD group: n = 35 additional IS (at day 28, n = 18 failures; at day 200, n = 33 failures, n = 2 not evaluable).
The proportion of UGI aGVHD failures with progression to LGI aGVHD was similar in the BDP and BDP+BUD arms. At day 28, we observed 26 local failures in patients treated with BDP alone, with 11 (42%) progressions to LGI, whereas after treatment with BDP+BUD, we recorded 15 local failures, with 8 (53%) progressions to LGI (P= .4). At day 200, we observed 3 LGI progressions after BDP versus 5 LGI progressions with BDP +BUD (P= .7).
cGVHD
The cumulative incidence of cGVHD of any severity was 51 (67%) in the BDP arm versus 58 (73%) in the BDP+BUD arm (P= .2). The incidence of moderate to severe cGVHD was 53% with BDP and 45% with BDP+BUD.
CMV Reactivation
No significant differences were observed in CMV infection or disease in the period between UGI aGVHD onset and day 365 after the start of treatment, with a total of 23 patients (30%) with BDP versus 20 (25%) with BDP+BUD (P= .4). After BDP treatment, CMV reactivation occurred in 16 patients (70% of the total cases of CMV detection), and CMV disease occurred in 7 (30%). After BDP+BUD treatment, active CMV reactivation occurred in 17 patients (85%), and CMV disease occurred in 3 (15%). Among the patients with UGI aGVHD who were successfully treated with local GI agents alone and never received systemic steroids by day 200, we observed 10 (43%) cases of CMV infection or disease in the BDP group versus 11 (55%) in the BDP+BUD group.
OS
A suggestion of better OS was observed in patients treated with BDP+BUD compared with those treated with BDP alone; the 5-year OS was 51% (median not reached) for the BDP arm versus 57% (median not reached) for the BDP+BUD arm (P= .034) (Figure 3). The suggestion of better outcome with BDP +BUD was found on univariate analysis using the Cox model (HR, .6; 95% CI, .4 to 1.5; P= .07), but the difference was not significant in the multivariate model (Supplementary Table S1).
Figure 3.
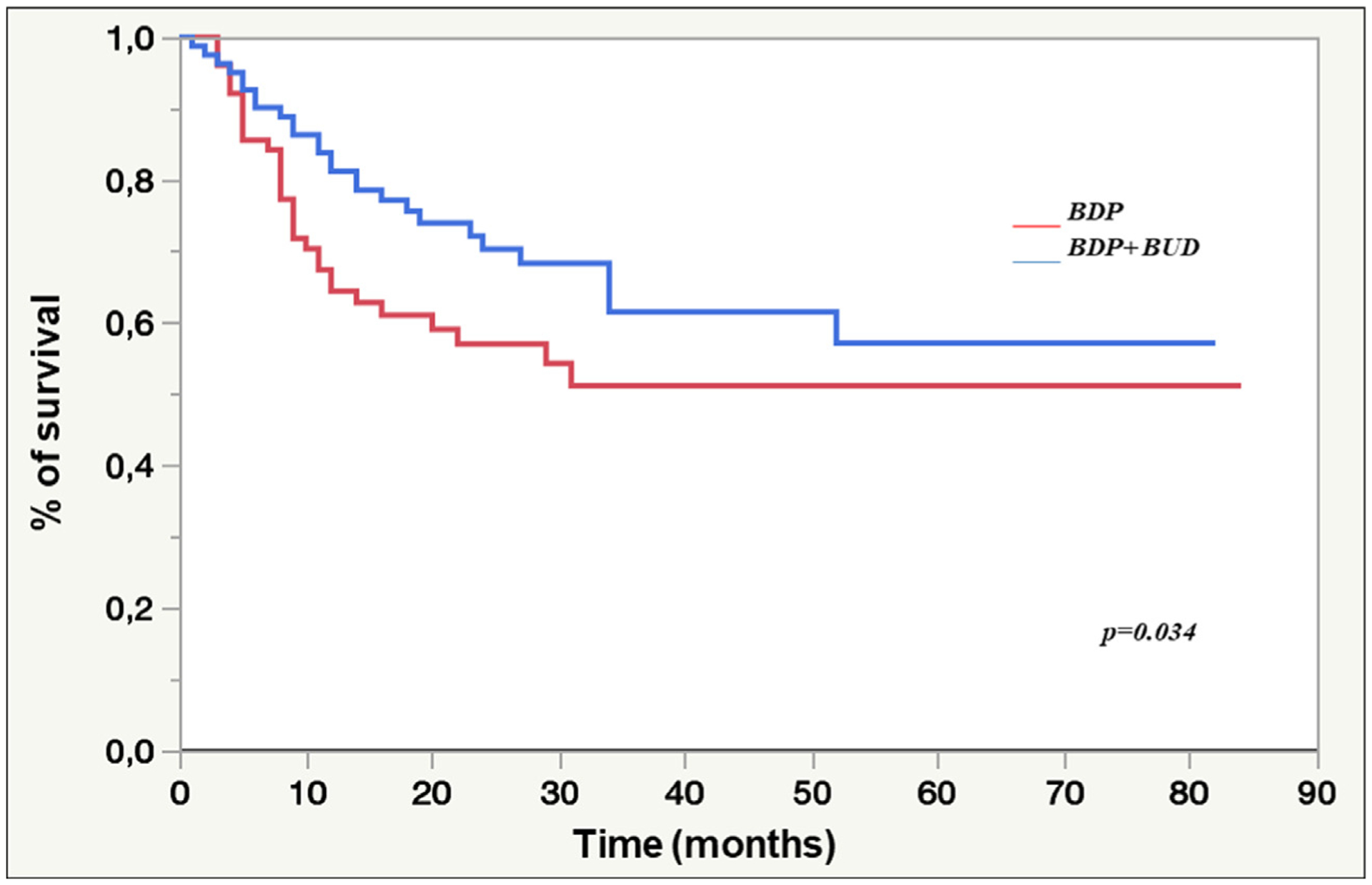
OS in the BDP and BDP+BUD cohorts. The 5-year OS rates did not differ in the multivariate model.
Causes of Death
The cause of death was transplantation-related mortality (TRM) in 23 patients (39%), recurrence or persistence of hematologic disease (non-TRM) in 32 patients (53%), and uncertain in 5 patients (8%). We observed 32 deaths (42%) in the BDP group versus 28 (35%) in the BDP+BUD group (P= .1). Analysis of causes of death revealed 9 TRM, 21 non-TRM, and 2 unknowns in the BDP group versus 14 TRM, 11 non-TRM, and 3 unknowns in BDP+BUD group (P= .04). Details of causes of death are provided in Table 3.
Table 3.
Causes of Death
| Cause of Death | BDP (n = 76) | BDP+BUD (n = 81) | P Value |
|---|---|---|---|
| TRM, n (%)* | 9(28) | 14(50) | .04 |
| ARDS, n (%) | 1(3) | 0(0) | |
| Cardiac failure, n (%) | 0(0) | 1(4) | |
| cGVHD, n (%) | 1(3) | 5(18) | |
| Pulmonary hemorrhage, n (%) | 2(6) | 0(0) | |
| Infection, n (%) | 3(10) | 5(18) | |
| Pneumonia, n (%) | 0(0) | 1(4) | |
| Previous malignancy, n (%) | 1(3) | 0(0) | |
| Pulmonary failure, n (%) | 1(3) | 1(4) | |
| Pulmonary, n (%) | 0(0) | 0(0) | |
| Renal failure, n (%) | 0(0) | 1(4) | |
| Non-TRM, n (%)* | 21 (66) | 11(39) | |
| Unknown, n (%)* | 2(6) | 3(11) | |
| Total, n (%)† | 32 (42) | 28(35) | .1 |
Significant P values (< .05) are in bold type.
TRM indicates transplantation-related mortality; non-TRM, recurrence/persistence/progression of hematologic disease at time of transplantation; ARDS, acute respiratory distress syndrome.
Percentages refer to the total number of deaths (n = 32 in BDP, n = 28 in BDP+BUD).
Percentages refer to the total number of patients by treatment group (n = 76 in BDP, n = 81 in BDP+BUD).
DISCUSSION
The present study reports the feasibility and efficacy of BDP with or without BUD for treatment of aGVHD affecting solely the UGI tract. Overall, 42% of patients were successfully treated without additional glucocorticoids and were spared the related toxicity. Our study is based on a relatively large, homogeneous, and well-characterized cohort of 157 patients with symptoms of isolated UGI aGVHD treated at a single transplantation center, and the results are sufficiently robust to justify the design of a controlled trial of BDP+BUD against systemic glucocorticoids. The response rate to poorly absorbable steroids BDP or BDP+BUD in this study is comparable to the response rate after systemic corticosteroids in a selected population of patients with isolated UGI aGVHD. After 28 days of treatment, we observed 63% CR+PR in the BDP arm and 77% CR +PR in the BDP+BUD arm, compared with 78% CR+PR with systemic prednisone at 2 mg/kg/day reported by McMillan et al [34]. Weisdorf et al [31] reported durable CR in 71% of their patients treated with systemic glucocorticoids. An older study reported a durable treatment response at day 30 of 41% with systemic prednisone at 1 mg/kg/day [19]. Within the constraints of a retrospective study, the response to treatment with poorly absorbable glucocorticoids appears to be comparable to that obtained with systemic glucocorticoids; overall, 42% of patients were successfully treated without additional systemic glucocorticoids and were spared the related toxicity. For patients with standard- risk aGVHD and isolated UGI involvement, we suggest considering an up-front approach using poorly absorbable glucocorticoids with similar efficacy and lower toxicity than standard systemic glucocorticoids.
A recent analysis of the Center for International Blood and Marrow Transplant Research database did not show a significant prognostic impact of UGI aGVHD on transplantation-related outcomes [29]. According to the Minnesota aGVHD risk classification, all patients in our present study cohort would be classified as standard risk [34,35]. These findings support the need for a risk-stratified therapy for patients with UGI aGVHD. We believe that an up-font approach with lower-intensity therapy with poorly absorbable BDP with or without BUD, avoiding systemic steroids up front, is consistent with the idea of tailoring lower-intensity therapy approaches for patients with standard-risk aGVHD and should be investigated in patients with isolated UGI aGVHD.
To the best of our knowledge, there are no published reports on the association of BDP and BUD without systemic glucocorticoids for treating aGVHD isolated to the UGI tract. In the present study, the major advantage of adding BUD to BDP was the higher initial response rate, despite the preponderance of high-risk patients in the BDP+BUD group. After 28 days of treatment, we found a suggestion of a higher response rate to BDP+BUD compared with BDP alone, detected in univariate analysis (P= .049) but not confirmed in multivariate analysis. After 200 days of treatment, 46% of patients treated with BDP +BUD and 34% of patients treated with BDP alone maintained a CR of UGI aGVHD without additional treatment (P not significant). Patients in the BDP+BUD group who failed to achieve CR or PR were more frequently treated with systemic glucocorticoids as a salvage treatment compared with patients in the BDP arm, with no impact on early death. There was also a suggestion of better OS with BDP+BUD compared to BDP alone, with a HR of .6 in univariate analysis (P= .07), but this was not seen in multivariate analysis. Progression to LGI aGVHD was equally distributed between the 2 groups; BUD seemed to improve GVHD control via systemic absorption along with the local control of GI GVHD. We did not observe any significant differences between the 2 treatment arms in secondary endpoints, including CMV reactivation, cGVHD incidence, and number of deaths; despite the small number of patients, there was a suggestion of higher TRM prevalence in the BDP+BUD arm.
Imbalance in baseline characteristics, with a preponderance of patients with high-risk UGI aGVHD allocated by physician choice to the BDP+BUD arm, might have contributed to the lack of superiority of BDP+BUD compared with BDP alone. Patients with earlier UGI aGVHD onset, those with histologically proven UGI aGVHD, and those treated with a myeloablative conditioning regimen (all features contributing to a high risk for treatment failure) were preferentially allocated to the BDP+BUD arm. The prevalence of deaths due to TRM in the BDP+BUD arm supports this hypothesis. Fewer patients treated with BDP+BUD underwent transplantation from an unrelated or HLA-incompatible donor. Other baseline characteristics, including demographic variables, primary disease type, disease risk category at HCT, stem cell source, aGVHD prophylaxis, and CMV recipient-donor relationship, were well balanced between the 2 treatment groups. In particular, the proportion of patients receiving a sirolimus- and methotrexate-containing regimen for aGVHD prophylaxis was similar in the 2 study groups, and there was no difference in OR at day 28 by GVHD treatment; the difference between sirolimus and methotrexate was evident when considering all patients together independent of aGVHD treatment.
Other approaches to limit the use of high-dose steroids to treat GVHD have been studied. Sirolimus used as up-front treatment in patients with standard-risk aGVHD was found to spare systemic glucocorticoid exposure and associated toxicity and to improve patients’ quality of life without a negative impact on long-term outcomes [36]. At the time of the present study, neither ruxolitinib nor ibrutinib had been approved for GVHD treatment, and neither drug was used at our center as an option for patients who failed BDP or BDP+BUD treatment. With the recent FDA approval of ruxolitinib for steroid-refractory aGVHD [37] and of ibrutinib for second-line or subsequent treatment for cGVHD [38], new trials are likely to explore the use of both agents for the initial treatment of aGVHD. Prospective studies should address other open questions, including the role of the microbiome in the development of GI GVHD, the use of post-transplantation cyclophosphamide to mitigate the severity of GI GVHD, and the role of GVHD biomarkers (ie, REG3 alpha and ST2) as indicators to escalate the intensity of immunosuppressive therapy [39,40]. In addition, BDP+BUD could be used in combination with low-dose systemic glucocorticoids in an attempt to improve GI aGVHD control and minimize side effects. The benefit of adding BDP to systemic glucocorticoids has been demonstrated in patients with both UGI and LGI GVHD; in a randomized trial, Hockenbery et al [18] reported a reduced risk of GVHD and mortality in patients treated with this combination; however, the concomitant systemic glucocorticoid administration obscured the detectable activity of BDP and might have contributed to the lack of FDA approval. Lacking a commercial formulation, some centers might not be able to dispense BDP because of the required compounding; a possible solution could be provided by a centralized drug supply for each state.
Notwithstanding the limits of this retrospective study, we conclude that sole treatment with poorly absorbable glucocorticoids is feasible and effective for patients with isolated UGI aGVHD, avoiding the toxicity of systemic glucocorticoids. Combining relatively nontoxic, topical therapy to minimize the use of, and thus the adverse effects of, systemic immunosuppression makes BDP+BUD an attractive combination therapy for UGI aGVHD management. Prospective trials should compare the safety and efficacy of BDP+BUD and systemic glucocorticoids in the treatment of patients with aGVHD isolated to the UGI tract.
Supplementary Material
ACKNOWLEDGMENTS
Financial disclosure: This study was supported in part by grant P30 CA076292 from the National Cancer Institute, National Insitutes of Health, Bethesda, MD.
Footnotes
Conflict of interest statement: There are no conflicts of interest to disclose.
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.bbmt.2020.04.023.
REFERENCES
- 1.Martin PJ, McDonald GB, Sanders JE, et al. Increasingly frequent diagnosis of acute gastrointestinal graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10:320–327. [DOI] [PubMed] [Google Scholar]
- 2.Martino R, Romero P, Subirá M, et al. Comparison of the classic Glucksberg criteria and the IBMTR Severity Index for grading acute graft-versus-host disease following HLA-identical sibling stem cell transplantation. International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1999;24:283–287. [DOI] [PubMed] [Google Scholar]
- 3.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 4.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. [DOI] [PubMed] [Google Scholar]
- 5.Takatsuka H, Iwasaki T, Okamoto T, Kakishita E. Intestinal graft-versus-host disease: mechanisms and management. Drugs. 2003;63:1–15. [DOI] [PubMed] [Google Scholar]
- 6.Van Lint MT, Milone G, Leotta S, et al. Treatment of acute graft-versus-host disease with prednisolone: significant survival advantage for day 5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood. 2006;107:4177–4181. [DOI] [PubMed] [Google Scholar]
- 7.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76:1464–1472. [PubMed] [Google Scholar]
- 8.Chao NJ, Chen BJ. Prophylaxis and treatment of acute graft-versus-host disease. Semin Hematol. 2006;43:32–41. [DOI] [PubMed] [Google Scholar]
- 9.Cutler C, Antin JH. Novel drugs for the prevention and treatment of acute GVHD. Curr Pharm Des. 2008;14:1962–1973. [DOI] [PubMed] [Google Scholar]
- 10.Khaled Y, Reddy P, Krijanovski O. Emerging drugs for acute graft-versus-host disease. Expert Opin Emerg Drugs. 2009;14:219–232. [DOI] [PubMed] [Google Scholar]
- 11.Perez L, Anasetti C, Pidala J. Have we improved in preventing and treating acute graft-versus-host disease? Curr Opin Hematol. 2011;18: 408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald GB. Oral beclomethasone dipropionate: a topically active corticosteroid for the treatment of gastrointestinal graft-versus-host disease following allogeneic hematopoietic cell transplantation. Expert Opin Investig Drugs. 2007;16:1709–1724. [DOI] [PubMed] [Google Scholar]
- 13.Fedorak RN, Bistritz L. Targeted delivery, safety, and efficacy of oral enteric-coated formulations of budesonide. Adv Drug Deliv Rev. 2005;57:303–316. [DOI] [PubMed] [Google Scholar]
- 14.Nunes T, Barreiro-de Acosta M, Nos P, et al. Usefulness of oral beclometasone dipropionate in the treatment of active ulcerative colitis in clinical practice: the RECLICU study. J Crohns Colitis. 2010;4:629–636. [DOI] [PubMed] [Google Scholar]
- 15.Tursi A, Giorgetti GM, Brandimarte G, Elisei W, Aiello F. Beclomethasone dipropionate for the treatment of mild-to-moderate Crohn’s disease: an open-label, budesonide-controlled, randomized study. Med Sci Monit. 2006;12:PI29–PI32. [PubMed] [Google Scholar]
- 16.Bar-Meir S, Chowers Y, Lavy A, et al. Budesonide versus prednisone in the treatment of active Crohn’s disease. the Israeli Budesonide Study Group. Gastroenterology. 1998;115:835–840. [DOI] [PubMed] [Google Scholar]
- 17.Caesar I, Gross V, Roth M, et al. Treatment of active and postactive ileal and colonic Crohn’s disease with oral pH-modified-release budesonide. German Budesonide Study Group. Hepatogastroenterology. 1997;44:445–451. [PubMed] [Google Scholar]
- 18.Hockenbery DM, Cruickshank S, Rodell TC, et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood. 2007;109:4557–4563. [DOI] [PubMed] [Google Scholar]
- 19.McDonald GB, Bouvier M, Hockenbery DM, et al. Oral beclomethasone dipropionate for treatment of intestinal graft-versus-host disease: a randomized, controlled trial. Gastroenterology. 1998;115:28–35. [DOI] [PubMed] [Google Scholar]
- 20.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. [DOI] [PubMed] [Google Scholar]
- 21.Bertz H, Afting M, Kreisel W, Duffner U, Greinwald R, Finke J. Feasibility and response to budesonide as topical corticosteroid therapy for acute intestinal GVHD. Bone Marrow Transplant. 1999;24:1185–1189. [DOI] [PubMed] [Google Scholar]
- 22.Ruutu T, Gratwohl A, de Witte T, et al. Prophylaxis and treatment of GVHD: EBMT-ELN Working Group recommendations for a standardized practice. Bone Marrow Transplant. 2014;49:168–173. [DOI] [PubMed] [Google Scholar]
- 23.Baehr PH, Levine DS, Bouvier ME, et al. Oral beclomethasone dipropionate for treatment of human intestinal graft-versus-host disease. Transplantation. 1995;60:1231–1238. [PubMed] [Google Scholar]
- 24.Castilla C, Pérez-Simón JA, Sanchez-Guijo FM, et al. Oral beclomethasone dipropionate for the treatment of gastrointestinal acute graft-versus-host disease (GVHD). Biol Blood Marrow Transplant. 2006;12:936–941. [DOI] [PubMed] [Google Scholar]
- 25.Iyer RV, Hahn T, Roy HN, et al. Long-term use of oral beclomethasone dipropionate for the treatment of gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:587–592. [DOI] [PubMed] [Google Scholar]
- 26.Miura Y, Narimatsu H, Kami M, et al. Oral beclomethasone dipropionate as an initial treatment of gastrointestinal acute graft-versus-host disease after reduced-intensity cord blood transplantation. Bone Marrow Transplant. 2006;38:577–579. [DOI] [PubMed] [Google Scholar]
- 27.Takashima S, Eto T, Shiratsuchi M, et al. The use of oral beclomethasone dipropionate in the treatment of gastrointestinal graft-versus-host disease: the experience of the Fukuoka Blood and Marrow Transplantation (BMT) group. Intern Med. 2014;53:1315–1320. [DOI] [PubMed] [Google Scholar]
- 28.Andree H, Hilgendorf I, Leithaeuser M, et al. Enteral budesonide in treatment for mild and moderate gastrointestinal chronic GVHD. Bone Marrow Transplant. 2008;42:541–546. [DOI] [PubMed] [Google Scholar]
- 29.Nikiforow S, Wang T, Hemmer M, et al. Upper gastrointestinal acute graft-versus-host disease adds minimal prognostic value in isolation or with other graft-versus-host disease symptoms as currently diagnosed and treated. Haematologica. 2018;103:1708–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakui M, Okamoto S, Ishida A, et al. Prospective evaluation for upper gastrointestinal tract acute graft-versus-host disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;23:573–578. [DOI] [PubMed] [Google Scholar]
- 31.Weisdorf DJ, Snover DC, Haake R, et al. Acute upper gastrointestinal graft-versus-host disease: clinical significance and response to immunosuppressive therapy. Blood. 1990;76:624–629. [PubMed] [Google Scholar]
- 32.Spencer GD, Hackman RC, McDonald GB, et al. A prospective study of unexplained nausea and vomiting after marrow transplantation. Transplantation. 1986;42:602–607. [DOI] [PubMed] [Google Scholar]
- 33.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease, I: Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2005;11:945–956. [DOI] [PubMed] [Google Scholar]
- 34.MacMillan ML, Robin M, Harris AC, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacMillan ML, DeFor TE, Weisdorf DJ. What predicts high risk acute graft-versus-host disease (GVHD) at onset?: identification of those at highest risk by a novel acute GVHD risk score. Br J Haematol. 2012;157:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pidala J, Hamadani M, Dawson P, et al. Randomized multicenter trial of sirolimus vs prednisone as initial therapy for standard-risk acute GVHD: the BMT CTN 1501 trial. Blood. 2020;135:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Przepiorka D, Luo L, Subramaniam S, et al. FDA approval summary: Ruxolitinib for treatment of steroid-refractory acute graft-versus-host disease [e-pub ahead of print]. Oncologist. doi: 10.1634/theoncolo-gist.2019-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miklos D, Cutler CS, Arora M, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130:2243–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mariotti J, Granata A, Bramanti S, et al. The new refined Minnesota risk score for acute graft-versus-host disease predicts overall survival and non-relapse mortality after T cell-replete haploidentical stem cell transplant with post-transplant cyclophosphamide. Bone Marrow Transplant. 2019;54:1164–1167. [DOI] [PubMed] [Google Scholar]
- 40.Mussetti A, Greco R, Peccatori J, Corradini P. Post-transplant cyclophosphamide, a promising anti-graft versus host disease prophylaxis: where do we stand. Expert Rev Hematol. 2017;10:479–492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


