Fig. 1.
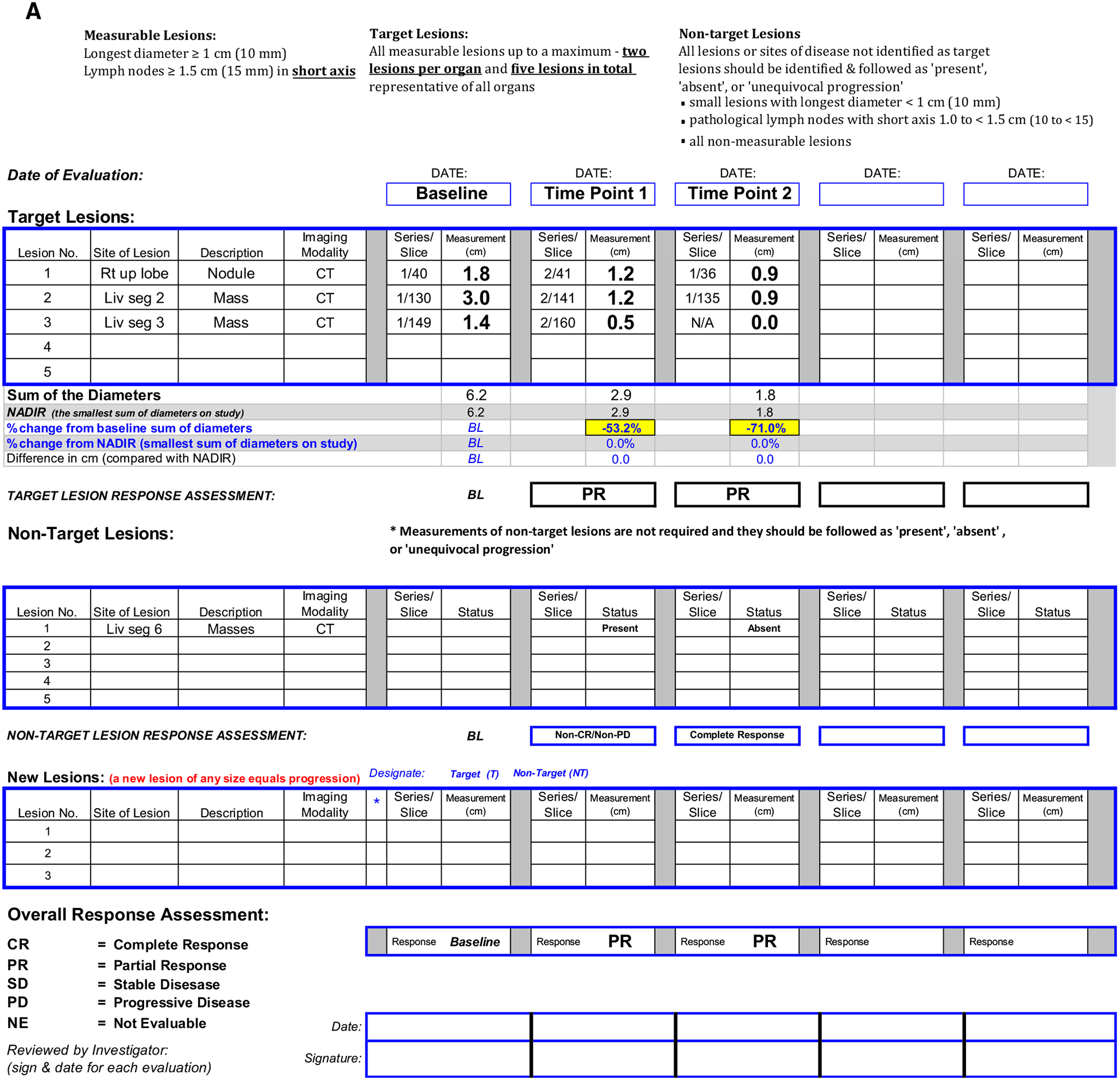
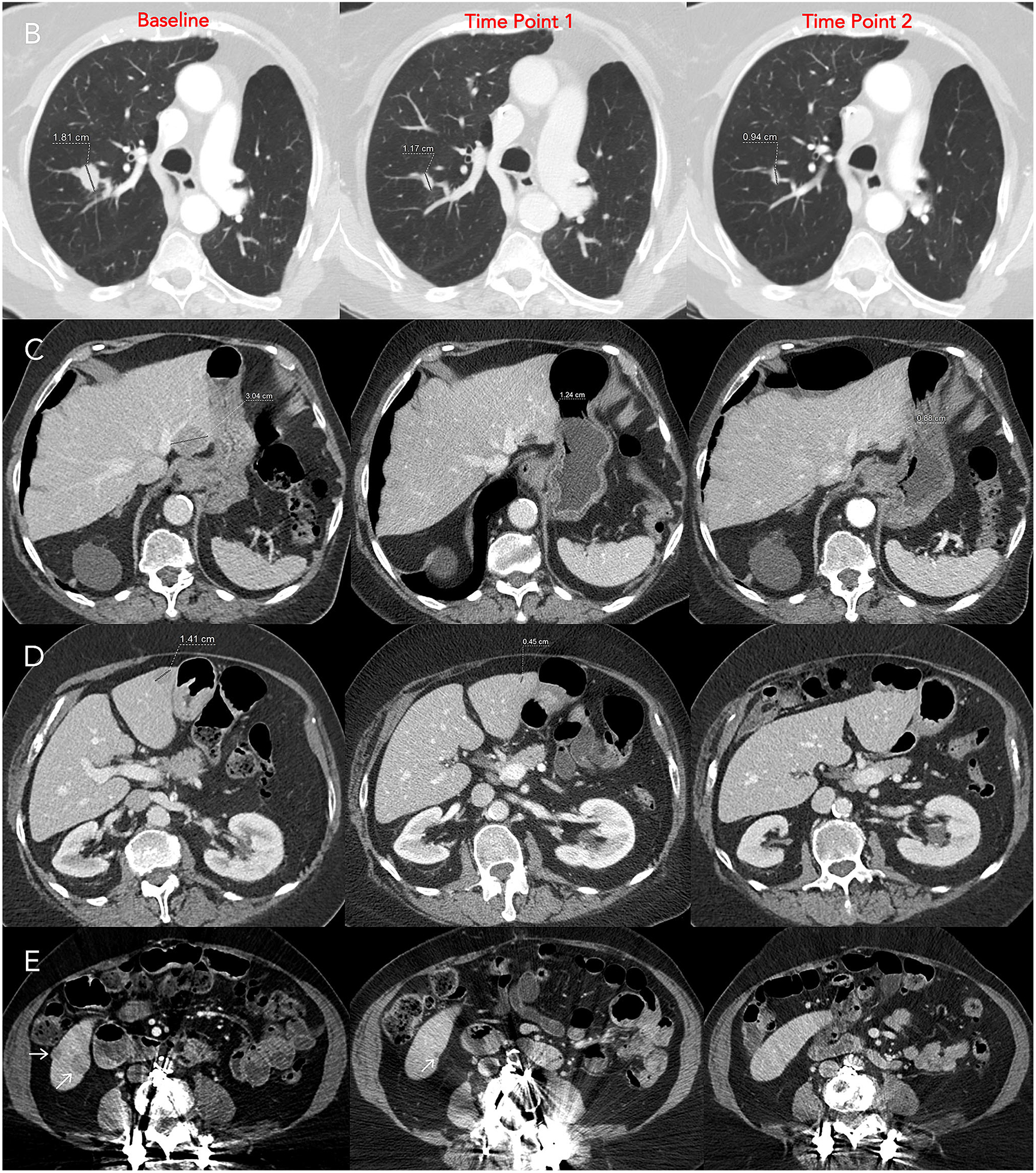
A Clinical trial worksheet using RECIST v1.1 criteria. B Target lesion 1, right upper lobe nodule, C Target lesion 2, segment 2 liver mass, D Target lesion 3, segment 3 liver mass, E Non-target lesion 1, segment 6 liver masses. Target lesions are assessed by summing the diameters at baseline and follow-up time points. In this case, the shrinkage of target lesions results in a partial response at both follow-up time points. Since two target liver lesions are assigned, the maximum number of target lesions in the liver has been reached. Therefore, the remaining liver lesions are assigned as non-target lesion 1.
