Abstract
Background.
Robotic pelvic lymphadenectomy (rPLND) has been demonstrated to be a safe and effective minimally invasive approach for patients with metastatic melanoma to the iliac nodes. However, the long-term oncologic benefit of this procedure remains poorly defined.
Methods.
A single-institutional study comparing perioperative outcomes and survival [recurrence-free (RFS) and overall survival (OS)] between rPLND and open PLND (oPLND) for metastatic melanoma was conducted.
Results.
From 2006 to 2018, a total of 63 PLND cases were identified: 22 rPLND and 41 oPLND. Evidence of isolated pelvic metastasis was the most common indication for PLND in both groups (rPLND: 64%, oPLND: 85%). There was no difference in median pelvic lymph node yield (11 vs. 9 nodes, p = 0.65). Neither treatment group experienced a Clavien-Dindo complication ≥ 3. rPLND was associated with a shorter length of stay compared with oPLND (2 vs. 4 days, p < 0.001). With a median follow-up of 37 months, there was no difference in RFS (14.4 vs. 9.6 months, p = 0.47) and OS (43 vs. 50 months, p = 0.58) between rPLND and oPLND, respectively. In basin recurrence was low with 1 (4.5%) and 3 (7.3%) patients in the rPLND and oPLND cohorts, respectively, experiencing an event (p = 0.9).
Conclusions.
rPLND for metastatic melanoma is a safe, minimally invasive treatment strategy that appears to result in similar intermediate term recurrence and survival rates as oPLND but shorter hospital stays.
Management of pelvic lymph nodes in cutaneous melanoma is an ongoing area of debate. Many studies have investigated the relationship of inguinal lymph node involvement to pelvic lymph nodes. Pelvic lymph nodes are involved in approximately 10–15% of patients with clinically occult positive inguinal lymph nodes, which increases to 30% when there is palpable inguinal lymphadenopathy.1–3 Studies also have found worse prognosis when pelvic lymph nodes are involved.1,4 Several studies have attempted to identify factors that predict pelvic lymph node involvement, but no definitive relationships have been established.4,5 The lack of predictive factors coupled with worse prognosis with pelvic node involvement forms the basis of the support for performing pelvic lymphadenectomy, albeit in select patients.
According to the National Comprehensive Cancer Network (NCCN) practice guidelines, pelvic lymph node dissection should be considered if there is radiologic evidence of iliac and/or obturator lymph node involvement, if Cloquet’s node is involved, for clinically positive inguinofemoral nodes, or if three or more inguinofemoral nodes are involved (NCCN v1.2019).6 Additional relative indications for PLND include large inguinal lymph node metastasis (≥ 3 cm) and a pelvic sentinel lymph node (SLN) identified on lymphoscintigraphy that was not sampled in the setting of a positive inguinal SLN.1,7
Robotic-assisted pelvic lymphadenectomy (rPLND) is now an established procedure routinely performed in urologic and gynecologic surgery with well-established safety.8–14 However, since the publication of our single-institution rPLND experience in 2016, there remains a paucity of publications discussing rPLND in the management of metastatic cutaneous melanoma.7 Sohn, Ross, and Pellegrino have reaffirmed our previous findings by publishing small case series that demonstrated rPLND to result in low complication rates and short hospital stays.15–18 To date, there are no published data comparing longer-term outcomes between rPLND and open oPLND. In this study, we present a relatively large series of patients undergoing rPLND and compare outcomes to a cohort of patients that underwent oPLND at a single, high-volume institution.
METHODS
Following approval by the institutional review board, a single-center, retrospective review was conducted that identified all patients with malignant melanoma who underwent a PLND from 2006 to 2018. Cases were included if PLND was performed alone or in conjunction with a superficial inguinal node dissection.
Clinicopathologic variables abstracted from hospital records included age, gender, body mass index (BMI), oncologic history (primary tumor location, Breslow depth, and sentinel lymph node biopsy (SLNB) results), and extent of pelvic lymph node dissection (overall pelvic lymph node yield, number of positive pelvic lymph nodes). Perioperative details included indications for surgery and operating room time, which was calculated as the difference between “in room” and “out of room” time, whereas operative time was calculated as the difference between “incision” and “close” time. Additional variables obtained were hospital length of stay, Clavien-Dindo complication score, and pattern of recurrence.
Patient cohorts were stratified based on operative approach (robotic vs. open). Decisions to proceed with PLND and the final operative approach were determined by the primary surgeon after discussion at a multidisciplinary cutaneous tumor board. Historically, obese patients were more likely to be considered for rPLND, whereas the presence of bulky pelvic lymph node metastasis or prior history of extensive abdominal or pelvic surgeries were more likely to proceed with oPLND. Preoperative staging included cross sectional imaging by way of computed tomography (CT) or whole body positron emission tomography/CT (PET/CT) combined with brain magnetic resonance imaging (MRI). Technical details for both rPLND and oPLND have been previously described.7
Continuous variables were presented as medians and interquartile range, whereas categorical variables were described as frequencies and proportions. Baseline characteristics between treatment cohorts were compared using the Kruskal-Wallis test and Chi square/Fisher’s exact test for continuous and categorical variables, respectively. Outcomes measured included recurrence-free survival (RFS) and overall survival (OS). Time to recurrence and death were calculated from the time of procedure. The Kaplan-Meier method was used to produce survival curves. A comparison of survival distributions was completed using the log-rank test. A two-sided alpha level was set at 0.05. All statistical analyses were performed using R version 3.5 and SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
A total of 63 patients underwent PLND during the study period: 22 rPLND versus 41 oPLND. Table 1 reflects the baseline clinicopathologic characteristics of the study cohort. The median patient age (rPLND: 64 vs. oPLND: 65, p = 0.79) and gender distribution (female: rPLND 59.1% vs. oPLND 65.9%, p = 0.8) between treatment groups were similar. Additionally, there was no difference in body mass index, location, and Breslow depth of the primary melanoma. PLND was combined with an open superficial inguinal dissection in 11 rPLND (50%) and 30 oPLND cases respectively (73.3%, p = 0.12).
TABLE 1.
Patient and tumor characteristics
| Variable | Overall (n = 63) | rPLND (n = 22) | oPLND (n = 41) | p value |
|---|---|---|---|---|
| Age, median (IQR) | 65 (53–75) | 64 (52–74.8) | 65 (53–75) | 0.79 |
| Sex [n (%)] | ||||
| Male | 23 (36.5) | 9 (40.9) | 14 (34.1) | 0.8 |
| Female | 40 (63.5) | 13 (59.1) | 27 (65.9) | |
| BMI [median (IQR)] | 29.1 (25.9–32) | 26.8 (22.3–31.8) | 29.3 (26.7–32.1) | 0.26 |
| Location of primary tumor [n (%)] | ||||
| Unknown | 9 (14.3) | 3 (13.6) | 6 (14.6) | 0.99 |
| Buttock/flank | 11 (17.5) | 4 (18.2) | 7 (17.1) | |
| Lower extremity | 43 (68.2) | 15 (68.2) | 28 (68.2) | |
| Breslow depth of primary tumor [mm; median (IQR)] | 3.5 (1.6–5.2) | 3.5 (2.4–5.5) | 3.5 (1.5–5.0) | 0.63 |
| Systemic therapy before PLND [n (%)] | 18 (28.6) | 7 (31.8) | 11 (26.8) | 0.9 |
| Systemic therapy after PLND [n (%)] | 40 (63.5) | 12 (54.5) | 28 (63.8) | 0.42 |
| Indication for pelvic lymph node dissection [n (%)] | 0.04 | |||
| Radiographic evidence/biopsy proven pelvic disease | 48 (76.2) | 14 (63.6) | 34 (82.9) | |
| Pelvic drainage on SLNB (not sampled) in setting of positive inguinal SLN | 9 (14.3) | 6 (27.3) | 3 (7.3) | |
| Involvement of 3 or more positive inguinal nodes | 4 (6.3) | 2 (9.1) | 2 (4.9) | |
| Large (> 3 cm) positive groin disease | 2 (3.2) | 0 | 2 (4.9) |
rPLND robotic pelvic lymph node dissection; oPLND open pelvic lymph node dissection; IQR interquartile range; BMI body mass index; PLND pelvic lymph node dissection; SLNB sentinel lymph node biopsy; SLN sentinel lymph node
In the present study, we identified four main primary indications for PLND, which included > 3 involved inguinal nodes, a large (≥ 3 cm) inguinal metastasis, pelvic sentinel node identified (but not sampled) on lymphoscintigraphy in the setting of a positive node in the superficial groin (pre-MSLT2), or radiologic- or biopsied-based evidence of metastatic disease involving pelvic nodes without evidence of distant disease (Table 1). In both groups, the most common indication for PLND was for radiologic/biopsy proven pelvic nodes (rPLND: 63.6% vs. oPLND: 83%, p = 0.042). Neoadjuvant therapy (interferon, targeted therapy, or immunotherapy) was given to 7 patients (31.8%) in the rPLND group versus 11 patients (26.8%) in the oPLND group before PLND (p = 0.9).
Perioperative Details
There was no significant difference in median operating room and operative time between treatment cohorts (Table 2). Hospital length of stay was significantly shorter for patients who underwent rPLND compared with oPLND (2 vs. 4 days, p < 0.001). Both operative approaches were well tolerated with no Clavien-Dindo grade III or higher complications occurring in either treatment group.
TABLE 2.
Perioperative outcomes
| Variable | Overall (n = 63) | rPLND (n = 22) | oPLND (n = 41) | p value |
|---|---|---|---|---|
| Combine procedure (open superficial inguinal dissection + o/r PLND) [n (%)] | 41 (65.1) | 11 (50) | 30 (73.2) | 0.79 |
| Operating room time, [minutes; median (IQR)] | 262 (222.2–292) | 267.5 (226.5–284.8) | 258.5 (197.5–299.2) | 0.49 |
| Surgery time, [min; median (IQR)] | 208.5 (155.5–243.2) | 199 (168–229.2) | 214.5 (151.5–250.2) | 0.76 |
| Complications by Clavien-Dindo score [n (%)] | 0.11 | |||
| 0 | 43 (68.3) | 12 (54.5) | 31 (75.6) | |
| 1 | 19 (30.2) | 9 (40.9) | 10 (24.4) | |
| 2 | 1 (1.5) | 1 (4.6) | 0 | |
| ≥ 3 | 0 | 0 | 0 | |
| Pelvic lymph node yield, median (IQR) | 10 (8–12) | 11 (8.2–12) | 9 (8–13) | 0.65 |
| Positive pelvic lymph nodes, median (IQR) | 1 (0–3) | 1 (0–1) | 2 (0–4) | 0.11 |
| Number of positive pelvic lymph nodes [n (%)] | 0.06 | |||
| 0 | 22 (34.9) | 10 (45.5) | 12 (29.3) | |
| 1 | 14 (22.2) | 7 (31.8) | 7 (17) | |
| >1 | 27 (42.9) | 5 (22.7) | 22 (53.7) | |
| Total positive regional nodes (pelvic + inguinal), median (IQR) | 2 (1–5) | 1 (0–4.5) | 2 (1–5.0) | 0.06 |
| Largest metastatic focus, [mm; median (IQR)] | 30 (20–42) | 18 (8.8–24.2) | 33 (27–49) | 0.002 |
rPLND robotic pelvic lymph node dissection; oPLND open pelvic lymph node dissection; IQR interquartile range; PLND pelvic lymph node dissection
Overall pelvic (iliac and obturator) lymph node yield following PLND was similar between rPLND and oPLND approaches (11 vs. 9 lymph nodes, p = 0.65). The median number of positive pelvic lymph nodes on final pathology was 1 and 2 for rPLND and oPLND, respectively (p = 0.11). However, of those patients who had a positive pelvic lymph node, the median, largest metastatic tumor deposit was larger in the oPLND compared with the rPLND cohort (33 mm vs. 18 mm, p = 0.002).
Survival
With a median follow-up of 37.2 months (IQR: 19.2–60 months), there was no significant difference in RFS (median RFS: 14.4 vs. 9.6 months, p = 0.47; Fig. 1a) and OS (median OS: 42.6 vs. 50 months, p = 0.58; Fig. 1b) between patients that underwent rPLND vs oPLND respectively. To date, patterns of recurrence did not differ between groups. Development of distant disease was the most common presentation following either operative approach (rPLND: 40.9% vs. oPLND: 43.9%, p = 0.93). In-basin recurrence was low; only one patient (4.5%) in the rPLND group and three patients (7.3%) in the oPLND experienced an event.
FIG. 1.
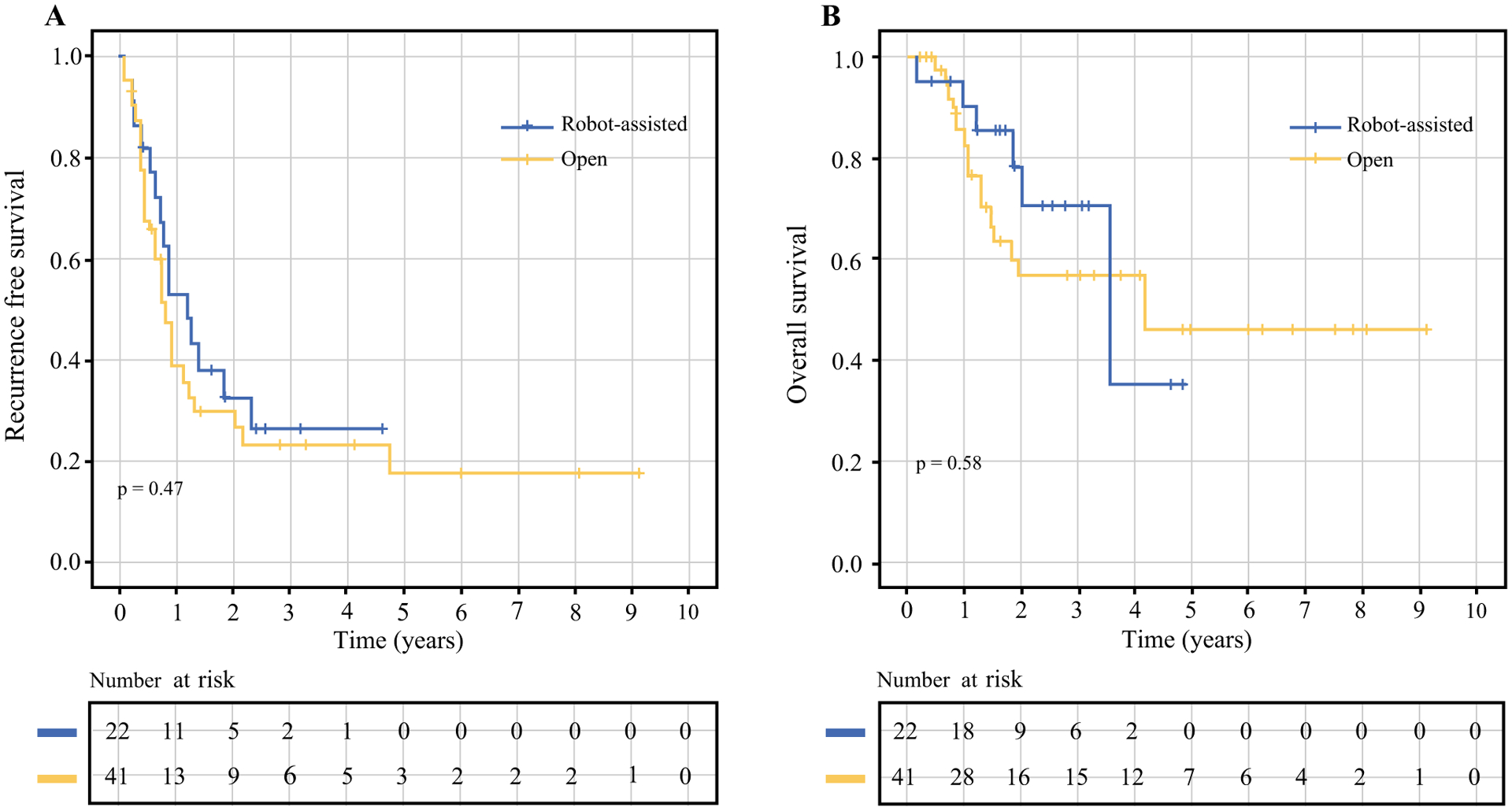
a Recurrence-free survival and b overall survival (in months) following pelvic lymph node dissection, stratified by operative approach (open vs. robotic-assisted). There was no significant difference in either recurrence free or overall survival between operative approaches to metastatic disease in the pelvic lymph nodes
DISCUSSION
As the treatment paradigms for melanoma continue to change, especially following the introduction of effective immunotherapy and targeted agents, surgical practice for this disease is also going through its own evolution. We previously reported our institution’s early outcomes with rPLND for melanoma and demonstrated this approach to be safe and effective. In the present study, we have since expanded our experience to include longer term follow-up and have determined that rPLND results in similar oncologic outcomes in both RFS and OS compared with the traditional oPLND for melanoma.
Following the introduction of robotic surgery, there has been a dramatic expansion of this technology throughout the oncology community. Recent application of this minimal invasive technique has been integrated into melanoma surgical approaches but has primarily been applied to the management of regional nodal basins. However, the advantages of robotic surgery seen in other disease sites also have been preserved when performed for melanoma. A notable finding is the significant decrease in hospital length of stay for patients undergoing a rPLND compared with the open cohort (2 vs. 4 days, p ≤ 0.001). Complication rates were also rare with no Clavien grade 3 or higher event occurring. While we did not capture postoperative pain scores or time to initiation of adjuvant therapies, the shorter LOS with rPLND (median, 2 days) also resulted in a cost savings of roughly $5625 per patient, as demonstrated in our prior study.7
Additionally, the technical success following rPLND, which can be defined by lymph node yield, remains promising. Small case series report a mean number of lymph nodes retrieved after rPLND ranging from 5 to 24 lymph nodes.16–18 In our expanded analysis, lymph node retrieval through a robotic approach was similar to these reports but, most importantly, was not inferior to oPLND [median pelvic lymph node yield (IQR); rPLND 11 (8.2–12) vs. oPLND 9 (8–13), p = 0.65]. Furthermore, the median number of lymph nodes retrieved, whether by a robotic or open approach, was comparable to existing experiences that reported on quality assurance parameters when performing regional lymph node dissection. The study by Spillane et al. reported a median lymph node retrieval parameter of 12 when only a PLND was performed.19 This number is in line with the current study, suggesting adequate lymph node retrieval for included patients. Therefore, from a perioperative perspective, rPLND is well tolerated and technically does not compromise regional nodal clearance.
However, paramount to oncologic surgery is the overall impact it has on long-term outcomes. Local disease control often is the metric used to assess the success of a surgery. While evaluation of the regional nodal basin contributes to the staging and overall prognosis, PLND in select patients has the potential to render a patient disease-free and ultimately affect survival. Historically, oPLND has been performed to treat regional disease and continues to be the preferred approach at our center in select circumstances—bulky regional disease, prior history of extensive intraabdominal operations, or inability to tolerate pneumoperitoneum. The in-basin failure rate was used in the present study as a surrogate for locoregional disease control. Of the 22 rPLND performed, there was only one in basin recurrence (4%), which was similar to oPLND (p = 0.93). In contrast, systemic/distant failure (n = 27, 42.9%) occurred most frequently; a finding commonly reported in other studies examining PLND.20–22 Consistent with the finding of no difference in pattern of recurrence, there was no difference in RFS and OS between surgical approaches, suggesting rPLND to be as effective as oPLND for the management of metastatic melanoma (Table 3).
TABLE 3.
Pattern of recurrence
| N (%) | Overall (n = 63) | rPLND (n = 22) | oPLND (n = 41) | p value |
|---|---|---|---|---|
| Median follow-up, mo (IQR) | 37.2 (19.2–60) | 30 (19.2–55.2) | 49 (16.8–80.4) | 0.08 |
| In basin | 4 (6.3) | 1 (4.6) | 3 (7.3) | 0.93 |
| Local or in-transit | 10 (15.9) | 3 (13.6) | 7 (17.1) | |
| Distant disease | 27 (42.9) | 9 (40.9) | 18 (43.9) | |
| No evidence of disease | 22 (34.9) | 9 (40.9) | 13 (31.7) |
rPLND robotic pelvic lymph node dissection; oPLND open pelvic lymph node dissection
In the absence of clinically positive pelvic disease, routine PLND for melanoma remains a contentious topic. Currently, relative indications to perform PLND have included positive inguinal SLN or drainage to pelvic nodes on lymphoscintigraphy at the time of SLNB. The recent publications of the MSLT-II and DeCOG-SLT trials, both phase III randomized studies, have changed the role of completion lymph node dissection for the management of microscopic regional nodal disease.23,24 Both trials had congruent results, and demonstrated no difference in melanoma specific survival (MSS) in patients undergoing early completion lymph node dissection compared with observation for patients with SLN-positive disease (MSLT-II 3-year MSS: Dissection 86 ± 1.3% vs. Observation 86 ± 1.2%, p = 0.42). While PLND was not directly addressed in these trials, the results do question the added benefit of completion PLND in the absence of clinically relevant pelvic metastasis. Additional studies also have challenged the indications for PLND, demonstrating no improvement in survival when PLND is performed.20,25 Egger et al.20 reviewed patients from the Sunbelt Melanoma Trial and compared outcomes between SLN-positive patients who had a superficial groin dissection versus combined superficial and PLND. Among patients that had a PLND performed, they demonstrated no difference in DFS and OS between patients with tumor-positive and -negative pelvic lymph nodes. Therefore, in the setting of microscopic regional nodal disease, there continues to be a growing body of evidence to suggest the absence of a survival benefit with a more extensive lymphadenectomy. These results will likely impact the frequency in which one performs PLND as the indications to proceed with this procedure continues to evolve.
The subgroup of patients who continue to stand to benefit from PLND are those with enlarging radiographic and especially biopsy-proven pelvic disease that do not have evidence of distant disease. It has been demonstrated through population based analysis that the biologic behavior of regional lymph node metastasis belongs on a spectrum, with patients harboring clinically evident disease doing much worse than patients with clinically occult.26,27 Additionally, while pelvic lymph node metastasis might reflect involvement of second echelon nodes, it is still considered regional disease and thus needs to be considered with treatment planning.27 In a large, retrospective series evaluating PLND that included patients with clinically relevant pelvic disease, 5-year survival rates up to 43% have been reported, highlighting the ability to achieve a durable survival benefit in a subgroup harboring more aggressive locoregional disease.28 However, to evaluate the true benefit of PLND in this setting, trials would require enrolling patients with clinically positive pelvic nodes and randomizing one arm to observation, which many would deem unethical. In the present study, patient’s undergoing oPLND appeared to have more advanced disease as reflected by a greater frequency of patients undergoing surgery for radiographic evidence/biopsy proven disease, along with possessing larger tumor deposits within the positive pelvic lymph node. Despite possessing a higher disease burden, the oncologic outcomes were similar between the oPLND and rPLND cohorts, suggesting that PLND can still benefit patients with clinically relevant disease. Nonetheless, in the setting of radiographic or biopsy proven pelvic disease, we believe that PLND should be considered, especially if able to render a patient without evidence of disease. The surgical approach, whether performed robotically or open, appears to be similar in efficacy and therefore can be selected at the surgeon’s discretion.
There are several limitations inherent to any retrospective review. Despite the similarity in baseline clinicopathologic variables between study cohorts, the nonrandomized design is unable to account for confounding variables that may have impacted surgical approach, perioperative results, or long term outcomes. Patients with extensive, bulky pelvic lymph node disease and prior major abdominal surgeries were more likely to proceed with an open approach, thereby identifying a selection bias that may have affected outcomes. Furthermore, because the study period also spanned a time when new effective systemic therapies were approved, it is unclear how these treatments may have influenced survival outcomes for patients undergoing PLND (either open or robotic) in the recent years. We were unable to capture all specific therapies that patients may have received over their disease course (both before and after PLND), because it is likely that many had multiple lines of treatment.
The small treatment cohorts also limit the ability to perform well powered analysis or additional subgroup analysis. While conducting prospective, randomized, multicenter studies to expand the study cohort has the potential to mitigate these issues, executing new trials specifically comparing operative approaches (robotic vs. open) will be difficult as the number of PLND will likely decrease in the future as the indications for lymph node dissections continue to evolve. The fate of PLND, irrespective of approach, for the management of microscopic regional disease, also will be influenced by the results of the ongoing phase III, randomized EAGLE FM trial (NCT02166788), which is specifically evaluating the benefit of PLND for patients with positive inguinal lymph nodes and without evidence of pelvic node involvement. Nonetheless, when performed at high-volume cutaneous centers that also have expertise in robotic surgery, the present study demonstrates that rPLND can be performed safely and does not compromise oncologic outcomes.
REFERENCES
- 1.Mozzillo N, Pasquali S, Santinami M, et al. Factors predictive of pelvic lymph node involvement and outcomes in melanoma patients with metastatic sentinel lymph node of the groin: a multicentre study. Eur J Surg Oncol. 2015;41:823–9. [DOI] [PubMed] [Google Scholar]
- 2.Faut M, Kruijff S, Hoekstra HJ, et al. Pelvic lymph node dissection in metastatic melanoma to the groin should not be abandoned yet. Eur J Surg Oncol. 2018;44:1779–1785. [DOI] [PubMed] [Google Scholar]
- 3.Oude Ophuis CM, van Akkooi AC, Hoekstra HJ, et al. Risk factors for positive deep pelvic nodal involvement in patients with palpable groin melanoma metastases: can the extent of surgery be safely minimized? A retrospective, multicenter cohort study. Ann Surg Oncol. 2015;22 Suppl 3:S1172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niebling MG, Wevers KP, Suurmeijer AJ, et al. Deep lymph node metastases in the groin significantly affects prognosis, particularly in sentinel node-positive melanoma patients. Ann Surg Oncol. 2015;22:279–86. [DOI] [PubMed] [Google Scholar]
- 5.Swords DS, Andtbacka RHI, Bowles TL, et al. Routine retrieval of pelvic sentinel lymph nodes for melanoma rarely adds prognostic information or alters management. Melanoma Res. 2018. [DOI] [PubMed] [Google Scholar]
- 6.NCCN Clinical Practice Guidelines in Oncology: Melanoma. National Comprehensive Cancer Network, 2017. [Google Scholar]
- 7.Dossett LA, Castner NB, Pow-Sang JM, et al. Robotic-assisted transperitoneal pelvic lymphadenectomy for metastatic melanoma: early outcomes compared with open pelvic lymphadenectomy. J Am Coll Surg. 2016;222:702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandaglia G, Abdollah F, Hu J, et al. Is robot-assisted radical prostatectomy safe in men with high-risk prostate cancer? Assessment of perioperative outcomes, positive surgical margins, and use of additional cancer treatments. J Endourol. 2014;28:784–91. [DOI] [PubMed] [Google Scholar]
- 9.Herlemann A, Cowan JE, Carroll PR, et al. Community-based Outcomes of Open versus Robot-assisted Radical Prostatectomy. Eur Urol. 2018;73:215–23. [DOI] [PubMed] [Google Scholar]
- 10.Eifler JB, Cookson MS: Best evidence regarding the superiority or inferiority of robot-assisted radical prostatectomy. Urol Clin North Am. 2014;41:493–502. [DOI] [PubMed] [Google Scholar]
- 11.Suardi N, Larcher A, Haese A, et al. Indication for and extension of pelvic lymph node dissection during robot-assisted radical prostatectomy: an analysis of five European institutions. Eur Urol. 2014;66:635–43. [DOI] [PubMed] [Google Scholar]
- 12.Salehi S, Avall-Lundqvist E, Legerstam B, et al. Robot-assisted laparoscopy versus laparotomy for infrarenal paraaortic lymphadenectomy in women with high-risk endometrial cancer: a randomised controlled trial. Eur J Cancer. 2017;79:81–89. [DOI] [PubMed] [Google Scholar]
- 13.Ponce J, Barahona M, Pla MJ, et al. Robotic transperitoneal infrarenal para-aortic lymphadenectomy with double docking: technique, learning curve, and perioperative outcomes. J Minim Invasive Gynecol. 2016;23:622–7. [DOI] [PubMed] [Google Scholar]
- 14.Coronado PJ, Fasero M, Magrina JF, et al. Comparison of perioperative outcomes and cost between robotic-assisted and conventional laparoscopy for transperitoneal infrarenal paraaortic lymphadenectomy (TIPAL). J Min Invasive Gynecol. 2014;21:674–81. [DOI] [PubMed] [Google Scholar]
- 15.Sohn W, Finley DS, Jakowatz J, et al. Robot-assisted laparoscopic transperitoneal pelvic lymphadenectomy and metastasectomy for melanoma: initial report of two cases. J Robot Surg. 2010;4:129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross AD, Kumar P, Challacombe BJ, et al. The addition of the surgical robot to skin cancer management. Ann R Coll Surg Engl. 2013;95:70–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellegrino A, Damiani GR, Strippoli D, et al. Robotic transperitoneal ilioinguinal pelvic lymphadenectomy for high-risk melanoma: an update of 18-month follow-up. J Robot Surg. 2014;8:189–91. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrino A, Damiani GR, Terruzzi M, et al. Robot-assisted laparoscopic transperitoneal deep pelvic lymphadenectomy for metastatic melanoma of the lower limb: initial report of four cases and outcomes at 1-year follow-up. Updates Surg. 2013;65:339–40. [DOI] [PubMed] [Google Scholar]
- 19.Spillane AJ, Haydu L, McMillan W, et al. Quality assurance parameters and predictors of outcome for ilioinguinal and inguinal dissection in a contemporary melanoma patient population. Ann Surg Oncol. 2011;18:2521–8. [DOI] [PubMed] [Google Scholar]
- 20.Egger ME, Brown RE, Roach BA, et al. Addition of an iliac/obturator lymph node dissection does not improve nodal recurrence or survival in melanoma. J Am Coll Surg. 2014;219:101–8. [DOI] [PubMed] [Google Scholar]
- 21.Romano E, Scordo M, Dusza SW, et al. Site and timing of first relapse in stage III melanoma patients: implications for follow-up guidelines. J Clin Oncol. 2010;28:3042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Essner R, Scheri R, Kavanagh M, et al. Surgical management of the groin lymph nodes in melanoma in the era of sentinel lymph node dissection. Arch Surg. 2006;141:877–82; discussion 882–4. [DOI] [PubMed] [Google Scholar]
- 23.Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376:2211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17:757–767. [DOI] [PubMed] [Google Scholar]
- 25.Karakousis GC, Pandit-Taskar N, Hsu M, et al. Prognostic significance of drainage to pelvic nodes at sentinel lymph node mapping in patients with extremity melanoma. Melanoma Res. 2013;23:40–6. [DOI] [PubMed] [Google Scholar]
- 26.Balch CM, Gershenwald JE, Soong SJ, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28:2452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badgwell B, Xing Y, Gershenwald JE, et al. Pelvic lymph node dissection is beneficial in subsets of patients with node-positive melanoma. Ann Surg Oncol. 2007;14:2867–75. [DOI] [PubMed] [Google Scholar]


