Figure 1: Characteristics of tumour response to cemiplimab as per independent central review.
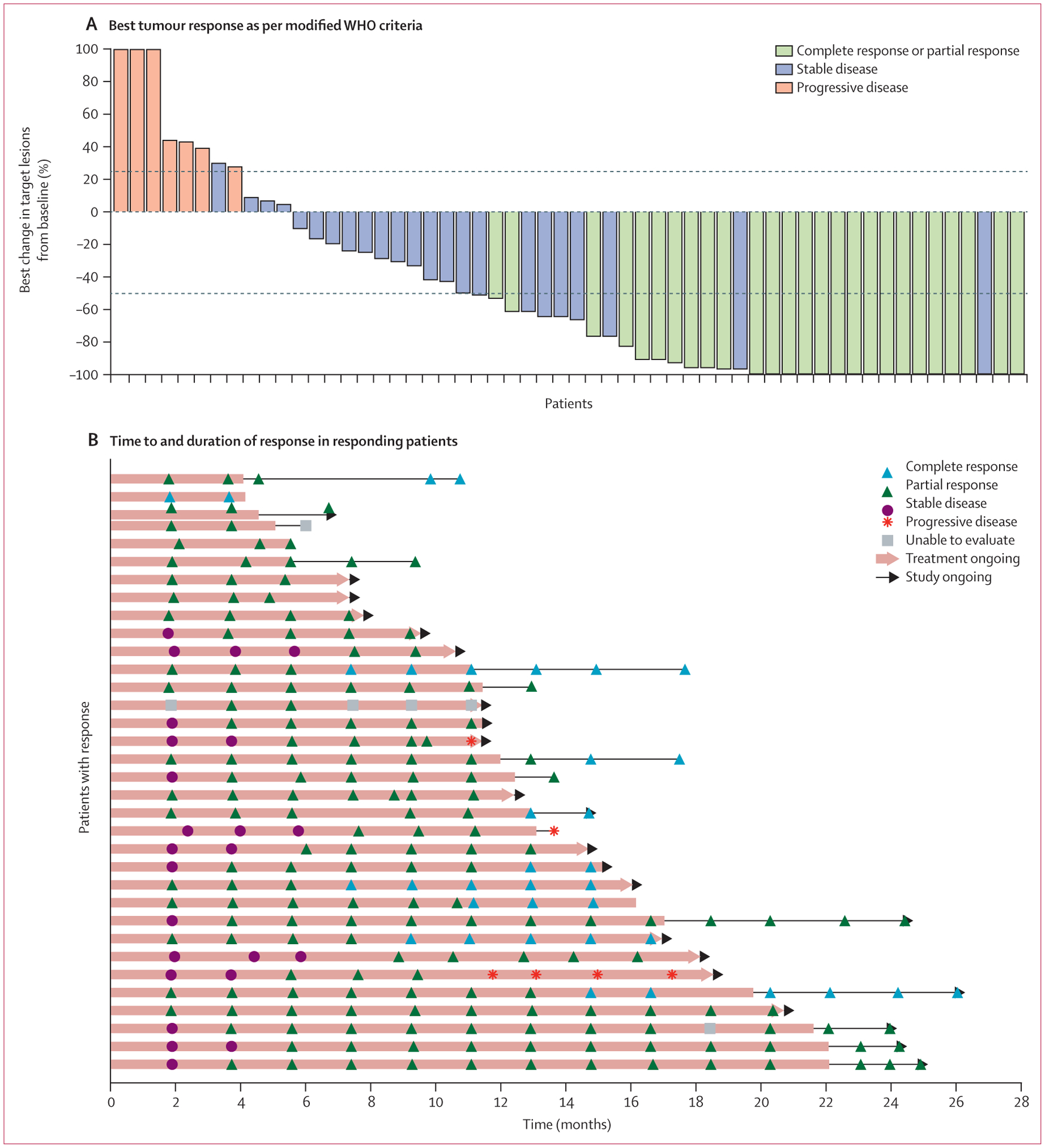
(A) Best change (percentage) in the sum of product (or products) of perpendicular longest dimensions of skin target lesion (or lesions) from baseline for 56 patients who had baseline skin target lesions and underwent at least one evaluable post-baseline medical photography evaluation as per modified WHO criteria by independent central review. Lesion measurements after progression were excluded. The dashed lines indicate WHO criteria for partial response (≥50% decrease in the sum of products of skin target lesion diameters) and progressive disease (≥25% increase in the sum of products of skin target lesion diameters). 22 patients who either did not have baseline skin target lesions or did not have evaluable post-baseline photography assessment are not included in the figure, but are included as non-responders in the overall response analysis as per the intention-to-treat principle. Eight patients had tumour reductions that met criteria for response on photographic measurements, but are classified as stable (blue bars >50% reduction in target lesions), either because there was no subsequent scan to confirm response (in seven patients) or because the composite response assessment was stable disease (in one patient). Eight of 34 patients with objective responses are not shown in this plot because the composite response assessments per independent central review included consideration of radiology results. (B) Time to response and duration of response in patients who responded to treatment. Each horizontal line represents one patient. Of the 34 responding patients, three had subsequent progressive disease. Among the remaining 31 patients who were still responding at the time of data cutoff, 12 were still on study treatment, nine were in post-treatment follow-up, and ten were off the study. One patient (sixth from bottom) had four progressive disease assessments because of discordance between investigator assessment and independent central review. Five patients with complete responses (top bar, and second, 12th, 17th, and 25th from top) are no longer on the study.
