Abstract
Background:
In the present study, we aim to develop simulation models based on computed tomography angiography images of intracranial aneurysms (IAs) and their parent vessels using three-dimensional (3D) printing technology. The study focuses on the value of these 3D models in presurgical planning and intraoperative navigation and ultimately their impact on patient outcomes. To the best of our knowledge, this is the first report of its kind from a war-torn country, like Iraq.
Methods:
This is a prospective study of a series of 11, consecutively enrolled, patients suffering from IAs for the period between February and September 2019. The study represents a collaboration between the two major neurosurgical centers in Baghdad/Iraq; Neurosciences Teaching Hospital and Neurosurgery Teaching Hospital. We analyzed the data of eleven patients with IAs treated by microsurgical clipping. These data include patient demographics, clinical, surgical, and outcomes along with the data of the 3D-printed replica used in these surgeries. All cases were operated on by one surgeon.
Results:
Our study included 11 patients, with a total of 11 aneurysms clipped. The mean age was 44 ± 8, with a median of 42.5 and a range of 35–61 years. About 60% of our patients were female with a female-to-male ratio of 1:5. About 60% of the aneurysms were located at the anterior communicating artery (Acom) while the remaining 40% were equally distributed between the posterior communicating and internal carotid arteries bifurcation. The standard pterional approach was followed in 50% of cases, whereas the other 50% of patients were treated through the lateral supraorbital approach. About 90% (n = 9) of the patients had a Glasgow Outcome Scale (GOS) of 5 and 10% had a GOS of 4. The 3D-printed models successfully replicated the aneurysm size, location, and relation to the parent vessel with 100% accuracy and were used for intraoperative guidance. The average production time was 24–48 h and the production cost was 10–20 US dollars.
Conclusion:
3D printing is a promising technology that is rapidly penetrating the field of neurosurgery. In particular, the use of 3D-printed patient-matched, anatomically accurate replicas of the cerebral vascular tree is valuable adjunct to the microsurgical clipping of IAs, and our study conclusions support this concept. However, both the feasibility and clinical utility of 3D printing remain the subject of much, ongoing investigations.
Keywords: Aneurysm surgery, Neurosurgery, Rapid prototyping, Three-dimensional printing

INTRODUCTION
Intracranial aneurysms (IAs) are localized dilatations in the cerebral vasculature with an inherently high risk of rupture, resulting in subarachnoid hemorrhage (SAH); a devastating form of hemorrhagic stroke. The overall prevalence of IAs in adults without specific risk factors for SAH is estimated to be between 2% and 3.2%.[1,24] The risk of SAH in subjects with IAs is 9/100,000/year.[2] The prehospital mortality rate for ruptured IAs is 10–15%. Among survivors, 12% will be severely disabled and 42% will be dependent, with the other 46% left with any combination of a range of disabilities.[3]
The management guidelines for both ruptured and unruptured IAs are continuously evolving. To date, the two main treatment modalities for IAs include microsurgical clipping and endovascular coil embolization. Several multicenter, randomized, controlled trials have compared the relative safety and efficacy of each treatment strategy; including the International Subarachnoid Aneurysm Trial, the Barrow Ruptured Aneurysm Trial, and the Prospective Registry of Subarachnoid Aneurysms Treatment, among others.[4-7] However, the absolute superiority of one treatment over another has not been established and selection of the treatment strategy should be individualized, taking into account both patient- and aneurysm-specific factors.[8]
As evident from the aforementioned trials, neurosurgical treatment for IAs is here to stay, despite the development of the more attractive, less invasive strategies. However, it is also true that the rate of intraoperative rupture (IOR) during IA clipping is almost quadruple that of endovascular coiling; 19% and 5%, respectively, as per the IA Rupture After Treatment study.[9] This alarmingly high IOR for microsurgical clipping has stirred interest in standardizing and personalizing treatment pathways for IAs. Such efforts may be exemplified by the introduction of the stereo imaging modalities and the three-dimensional (3D) printing technology.
The field of neurovascular surgery has been revolutionized by the introduction of modern stereo imaging modalities such as computed tomography angiography (CTA) and digital subtraction angiography (DSA). However, a significant gap between the details revealed by these conventional imaging modalities and the in vivo anatomy still exists;[10,11] this combined with the complexity and wide anatomical variations of IAs make judging the precise aneurysm geometry, orientation, size, and relation to the parent vessel challenging even to the more seasoned neurovascular surgeons.
In August 1984, Charles W Chuck filed a patent for “Apparatus for production of 3D objects by Stereolithography” and was subsequently accredited with the invention of the world’s first 3D printer (US Patent 4,575,330, 1986.).[12]
Over the subsequent decades, the 3D printing technology was enthusiastically embraced by several industries, with the health-care sector being at the forefront. The 3D printing technology relies on the principle of “additive manufacture” (AM), whereby successive layers of material are “printed” to create a 3D replica of a complex computer-designed model.
The main applications for 3D printing in surgery are (1) research and simulation-based teaching, (2) surgical planning, and (3) building tailor-made patient implants.[13] The 3D printing technology has mostly flourished in the field of orthopedic surgery, wherein custom-made implants and tissue bioprosthetics are being rapidly incorporated into the operating room.[14] On the other hand, the uptake of 3D printing in neurosurgery has progressed at a slower rate. Nonetheless, the use of 3D printing is mushrooming into the practice of neurosurgery, with an ever-growing number of game-changing applications.[15]
As an educational tool, 3D simulation models have been successfully trialed by multiple neurosurgery residency programs. These models offer an immersive virtual reality environment for junior neurosurgeons, enabling them to hone their skills in a controlled, standardized, and minimum risk setting.[13]
In the operating room, personalized, patient-matched, 3D models are being increasingly acknowledged as indispensable assets to the operating neurosurgeon. Throughout the operation, the ex vivo use of these models is highly valuable in maintaining the surgeon’s orientation, particularly concerning the aneurysm’s size, location, angulation, and parent vessel geometry; this is especially valuable in cases that involve complex and variant vascular anatomy.[16-22]
In the present study, we aim to develop simulation models based on CTA images of IAs and their parent vessels using 3D printing technology. The study focuses on the value of these 3D models in presurgical planning and intraoperative navigation and ultimately their impact on patient outcomes. To the best of our knowledge, this is the first report of its kind from Iraq.
MATERIALS AND METHODS
This is a prospective study of a series of 11, consecutively enrolled, patients suffering from IAs for the period between February and September 2019. The study represents a collaboration between the two major neurosurgical centers in Baghdad/Iraq; Neurosciences Teaching Hospital and Neurosurgery Teaching Hospital. We analyzed the data of 11 patients with IAs treated by microsurgical clipping. These data include patient demographics, clinical, surgical, and outcomes along with the data of the 3D-printed replica used in these surgeries. All cases were operated on by one surgeon.
The generation and postprocessing of CTA data
Dynamic CTA images procured. We used the following parameters, slice thickness, 1 mm; the field of view 240 mm.
Images exported in standard digital imaging (DICOM) format to the 3D calculation software (3D Slicer). The bones outside the field of interest, soft tissues, and brain tissue removed. The vascular and nearby bone area segmented by the software the virtual 3D angiogram generated. The skull, intracranial artery and aneurysm distinguished in different colors. Data from the segmented area exported in STL format to the XYZ printer software to produce a patient-specific prototype.
The manufacturing process of the 3D-printed models
We used two types of materials to produce the prototype. They were prerequisites by the printing machine itself. They were PLA and ABS filaments. These are semi-rigid and rigid, respectively. Eleven new models fabricated.
Preoperative planning
In the preoperative period, the vascular neurosurgeon suggested the ideal surgical approach for each model. The 3D models were used to simulate the route to reach the target aneurysm and to determine the size and shape of the clip for the aneurysms. The model-based preoperative plans were compared with the CTA-based, planned, surgical approach to examine the value of the model in preoperative planning.
RESULTS
Our study included 11 patients, with a total of 11 aneurysms clipped. The mean age was 44 ± 8, with a median of 42.5 and a range of 35–61 years. About 60% (n = 6) of our patients were female with a female-to-male ratio of 1.5. About 60% (n = 6) of the aneurysms were located at the Acom while the remaining 40% (n = 4) were equally distributed between the posterior communicating artery and the internal carotid artery bifurcation. The standard pterional approach for aneurysm clipping was followed in 50% (n=5) of cases, whereas the other 50% of patients (n = 5) were treated through the lateral supraorbital approach. Intraoperative aneurysm rupture occurred in 30% of the surgeries (n = 3). About 90% (n = 9) of the patients had a GOS of 5 and 10% (n = 1) had a GOS of 4. All surgeries were performed by the same surgeon. The 3D-printed models successfully replicated the aneurysm size, location, and relation to the parent vessel with a 100% accuracy and were used for intraoperative guidance [Figures 1 and 2]. The average production time was 24–48 h and the production cost was 10–20 US dollars.
Figure 1:
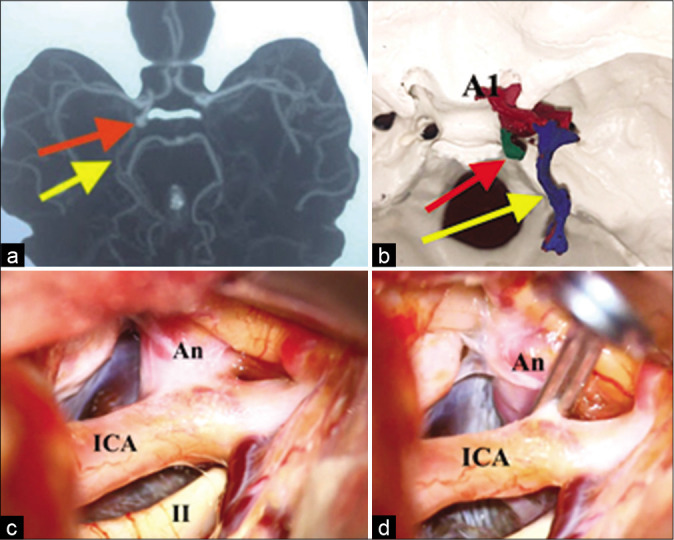
Images showing the comparison between: (a) the CT angiography image of (5 mm) posterolateral directed right posterior communicating artery aneurysm. The aneurysm (red arrow) and the basal vein of Rosenthal (yellow arrow). (b) 3D physical prototyped biomodel, (c) the intraoperative finding, before the clipping (c) and after the clipping (d). A1: Anterior cerebral artery, precommunicating segment. An: Aneurysm. ICA: Internal carotid artery. II: Optic nerve.
Figure 2:
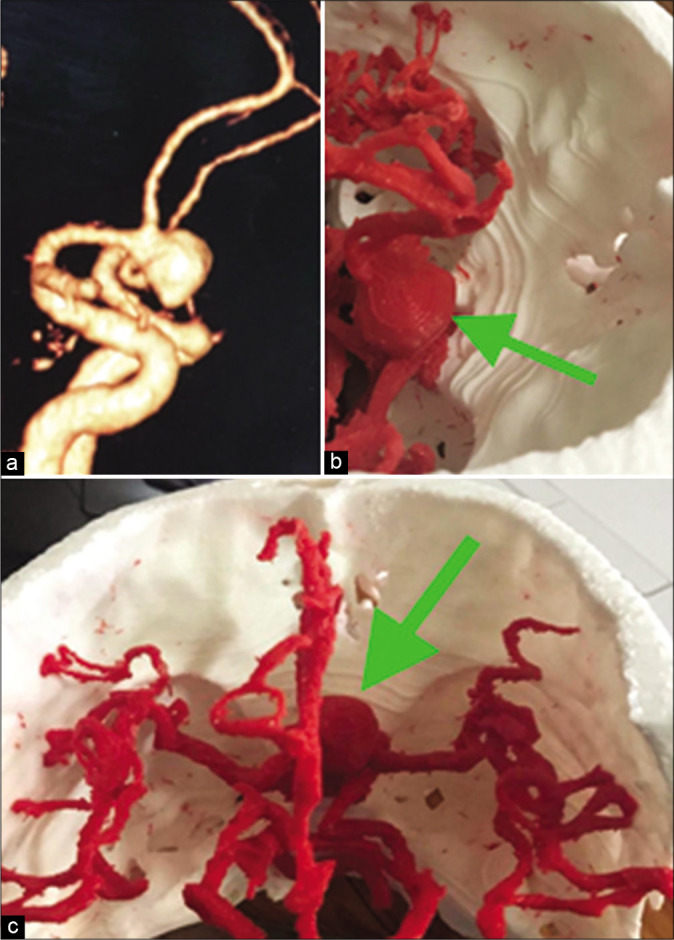
A case of large anterior communicating artery aneurysm. CT angiography three-dimensional (3D) reconstructed image (a). The 3D prototyped biomodel of the aneurysm, surrounding arteries and the skull base viewed from different angle to enhance preoperative planning and thus intraoperative orientation (b and c). Green arrow: Aneurysm.
DISCUSSION
The 3D printing technology is rapidly gaining popularity among neurosurgeons due to its promising potential to improve patient care. Although numerous proof-of-principle reports have confirmed the safety, efficacy, feasibility, accuracy, reproducibility, and cost-effectiveness of 3D printing in vascular neurosurgery,[16-22] there exist many hurdles to overcome before the technology is fully incorporated into routine neurosurgical and, more specifically, neurovascular practice. An important limitation is the lack of a standardized manufacturing pathway governing the assimilation of the 3D models; indeed, great variability exists in terms of the type of printers being used, the 3D printing technology at practice and, importantly, the characteristics of the resin materials employed in the synthesis process.
Our 3D-printed replicas exhibited good correlation with the real anatomy in all cases; a finding replicated in almost all studies that examined the use of 3D-printed constructs in vascular neurosurgery.[16-22] In our study, the anatomical accuracy of the 3D-printed replicas was judged visually, similar to the majority of other similar type studies where the authors have reported the anatomical agreement of their models with reference to visual comparisons between the anatomy observed at the time of surgery to the details exhibited by the models.[18,23,25] However, recent trends have been toward more objective, precise methods of validation; for example, sub-millimeter level measurements of the aneurysm and related vascular geometry were reported by Weinstock et al.[24] while Ionita et al.[5] reimaged the printed phantoms and compared the phantom DSA images to the source DSA data.[21] Furthermore, only the relation of the aneurysm to the parent vessel was examined in our study, with no emphasis on the smaller perforators.
It is also worth noting that, in this particular study, the source images were obtained using CTA data; none of the cases underwent DSA; this is in contrast to the majority of similar type studies where DSA was the primary source of images.[26,27] The use of CTA instead of DSA is an important limitation in our study as it significantly impacts the resolution threshold of the printed models. However, CTA is a more practical option, given the limited availability and clinical utility of DSA, when judged within the time constraint of acute SAH. Besides, in the majority of cases, nonvascular structures were excluded from the final model, except in a few cases where the aneurysm had an intimate relationship to the base of the skull; in these cases, the surrounding bony structures were included in the final replica to illustrate the relationship of the aneurysm to the adjacent skull structures like the clinoid processes.
The average manufacturing time was 24–48 h per model, measured from the obtainment of the CTA image to the last step in the production of the final 3D product. Zhao et al. reported an average manufacturing time of 20 h.[28] Time is often quoted as the main culprit holding back 3D printing, especially in cases of acute aneurysm rupture where time plays a pivotal role in the terminal outcome. Importantly, however, this perceived, time delay should be weighed against the benefits derived from shortening the intraoperative time and decreasing the number of unsuccessful clipping attempts.[24]
The type of materials we used, which were mandated by the type of the 3D printer, meant that the final constructs were too rigid and brittle to be compared to human vessels. Thus, the models were not amenable to the practice of vessel dissection and clipping. As our study aim focuses on the utility of the models in surgical guidance more so than training, the models were sufficient for our purposes. Nonetheless, using different resins can produce more flexible aneurysms that closely match reality with extended applicability for simulation and training purposes. A similar limitation was initially reported by Kang et al. who subsequently replicated their experiment to create models of flexible aneurysms enclosed in rigid skulls. According to the authors, these human-like models ultimately enabled an immersing virtual reality for surgeons allowing them to practice both craniotomy and clipping.[28] At the same time, the use of these materials does bring its own advantages including lower costs and faster production times.
CONCLUSION
3D printing is a promising technology that is rapidly penetrating the field of neurosurgery. In particular, the use of 3D-printed patient-matched, anatomically accurate replicas of the cerebral vascular tree is valuable adjunct to the microsurgical clipping of IAs, and our study conclusions support this concept. However, both the feasibility and clinical utility of 3D printing remain the subject of much, ongoing investigations.
Footnotes
How to cite this article: Faraj MK, Hoz SS, Mohammad AJ. The use of three-dimensional anatomical patient-specific printed models in surgical clipping of intracranial aneurysm: A pilot study. Surg Neurol Int 2020;11:381.
Contributor Information
Moneer K. Faraj, Email: drmkfaraj@gmail.com.
Samer S. Hoz, Email: hozsamer2055@gmail.com.
Amjad J. Mohammad, Email: amjadjasim76@gmail.com.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Anderson JR, Thompson WL, Alkattan AK, Diaz O, Klucznik R, Zhang YJ, et al. Three-dimensional printing of anatomically accurate, patient specific intracranial aneurysm models. J Neurointerv Surg. 2016;8:517–20. doi: 10.1136/neurintsurg-2015-011686. [DOI] [PubMed] [Google Scholar]
- 2.Clarke M. Systematic review of reviews of risk factors for intracranial aneurysms. Neuroradiology. 2008;50:653–64. doi: 10.1007/s00234-008-0411-9. [DOI] [PubMed] [Google Scholar]
- 3.Elijovich L, Higashida RT, Lawton MT, Duckwiler G, Giannotta S, Johnston SC, et al. Predictors and outcomes of intraprocedural rupture in patients treated for ruptured intracranial aneurysms: The CARAT study. Stroke. 2008;39:1501–6. doi: 10.1161/STROKEAHA.107.504670. [DOI] [PubMed] [Google Scholar]
- 4.Gölitz P, Struffert T, Knossalla F, Saake M, Ott S, Ganslandt O, et al. Angiographic CT with intravenous contrast injection compared with conventional rotational angiography in the diagnostic work-up of cerebral aneurysms. AJNR Am J Neuroradiol. 2012;33:982–7. doi: 10.3174/ajnr.A2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ionita CN, Mokin M, Varble N, Bednarek DR, Xiang J, Snyder KV, et al. Challenges and limitations of patient-specific vascular phantom fabrication using 3D Polyjet printing. Proc SPIE Int Soc Opt Eng. 2014;9038:90380M. doi: 10.1117/12.2042266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan IS, Kelly PD, Singer RJ. Prototyping of cerebral vasculature physical models. Surg Neurol Int. 2014;5:11. doi: 10.4103/2152-7806.125858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JA, Kim HN, Im SK, Chung S, Kang JY, Choi N. Collagen-based brain microvasculature model in vitro using three-dimensional printed template. Biomicrofluidics. 2015;9:024115. doi: 10.1063/1.4917508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondo K, Nemoto M, Masuda H, Okonogi S, Nomoto J, Harada N, et al. Anatomical reproducibility of a head model molded by a three-dimensional printer. Neurol Med Chir (Tokyo) 2015;55:592–8. doi: 10.2176/nmc.oa.2014-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanzino G, Murad MH, d’Urso PI, Rabinstein AA. Coil embolization versus clipping for ruptured intracranial aneurysms: A meta-analysis of prospective controlled published studies. AJNR Am J Neuroradiol. 2013;34:1764–8. doi: 10.3174/ajnr.A3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mashiko T, Otani K, Kawano R, Konno T, Kaneko N, Ito Y, et al. Development of three-dimensional hollow elastic model for cerebral aneurysm clipping simulation enabling rapid and low cost prototyping. World Neurosurg. 2015;83:351–61. doi: 10.1016/j.wneu.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 11.McDougall CG, Spetzler RF, Zabramski JM, Partovi S, Hills NK, Nakaji P, et al. The barrow ruptured aneurysm trial. J Neurosurg. 2012;116:135–44. doi: 10.3171/2011.8.JNS101767. [DOI] [PubMed] [Google Scholar]
- 12.Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–17. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 13.Namba K, Higaki A, Kaneko N, Mashiko T, Nemoto S, Watanabe E. Microcatheter shaping for intracranial aneurysm coiling using the 3-dimensional printing rapid prototyping technology: Preliminary result in the first 10 consecutive cases. World Neurosurg. 2015;84:178–86. doi: 10.1016/j.wneu.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Randazzo M, Pisapia JM, Singh N, Thawani JP. 3D printing in neurosurgery: A systematic review. Surg Neurol Int. 2016;7(Suppl 33):S801–9. doi: 10.4103/2152-7806.194059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rinkel GJ. Intracranial aneurysm screening: Indications and advice for practice. Lancet Neurol. 2005;4:122–8. doi: 10.1016/S1474-4422(05)00993-2. [DOI] [PubMed] [Google Scholar]
- 16.Serafin Z, Strześniewski P, Lasek W, Beuth W. Follow-up after embolization of ruptured intracranial aneurysms: A prospective comparison of two-dimensional digital subtraction angiography, three-dimensional digital subtraction angiography, and time-of-flight magnetic resonance angiography. Neuroradiology. 2012;54:1253–60. doi: 10.1007/s00234-012-1030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutradhar A, Park J, Carrau D, Miller MJ. Experimental validation of 3D printed patient-specific implants using digital image correlation and finite element analysis. Comput Biol Med. 2014;52:8–17. doi: 10.1016/j.compbiomed.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Taki W, Sakai N, Suzuki H, PRESAT Group. Determinants of poor outcome after aneurysmal subarachnoid hemorrhage when both clipping and coiling are available: Prospective registry of subarachnoid aneurysms treatment (PRESAT) in Japan. World Neurosurg. 2011;76:437–45. doi: 10.1016/j.wneu.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Vaishya R, Vaish A. Berlin: Springer; 2019. 3D Printing in Orthopedics; pp. 583–90. [Google Scholar]
- 20.Vanninen R, Koivisto T, Saari T, Hernesniemi J, Vapalahti M. Ruptured intracranial aneurysms: Acute endovascular treatment with electrolytically detachable coils-a prospective randomized study. Radiology. 1999;211:325–36. doi: 10.1148/radiology.211.2.r99ap06325. [DOI] [PubMed] [Google Scholar]
- 21.Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011;10:626–36. doi: 10.1016/S1474-4422(11)70109-0. [DOI] [PubMed] [Google Scholar]
- 22.Wang JL, Yuan ZG, Qian GL, Bao WQ, Jin GL. 3D printing of intracranial aneurysm based on intracranial digital subtraction angiography and its clinical application. Medicine (Baltimore) 2018;97:e11103. doi: 10.1097/MD.0000000000011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Ye X, Hao Q, Ma L, Chen X, Wang H, et al. Three-dimensional intracranial middle cerebral artery aneurysm models for aneurysm surgery and training. J Clin Neurosci. 2018;50:77–82. doi: 10.1016/j.jocn.2018.01.074. [DOI] [PubMed] [Google Scholar]
- 24.Weinstock P, Prabhu SP, Flynn K, Orbach DB, Smith E. Optimizing cerebrovascular surgical and endovascular procedures in children via personalized 3D printing. J Neurosurg Pediatr. 2015;16:584–9. doi: 10.3171/2015.3.PEDS14677. [DOI] [PubMed] [Google Scholar]
- 25.Whitaker M. The history of 3D printing in healthcare. Bull R Coll Surg Engl. 2014;96:228–9. [Google Scholar]
- 26.Wurm G, Tomancok B, Pogady P, Holl K, Trenkler J. Cerebrovascular stereolithographic biomodeling for aneurysm surgery. Technical note. J Neurosurg. 2004;100:139–45. doi: 10.3171/jns.2004.100.1.0139. [DOI] [PubMed] [Google Scholar]
- 27.Xu WH, Liu J, Li ML, Sun ZY, Chen J, Wu JH. 3D printing of intracranial artery stenosis based on the source images of magnetic resonance angiograph. Ann Transl Med. 2014;2:74. doi: 10.3978/j.issn.2305-5839.2014.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J, Lin H, Summers R, Yang M, Cousins BG, Tsui J. Current Treatment Strategies for Intracranial Aneurysms: An Overview. Angiology. 2018;69:17–30. doi: 10.1177/0003319717700503. [DOI] [PMC free article] [PubMed] [Google Scholar]


