Figure 1.
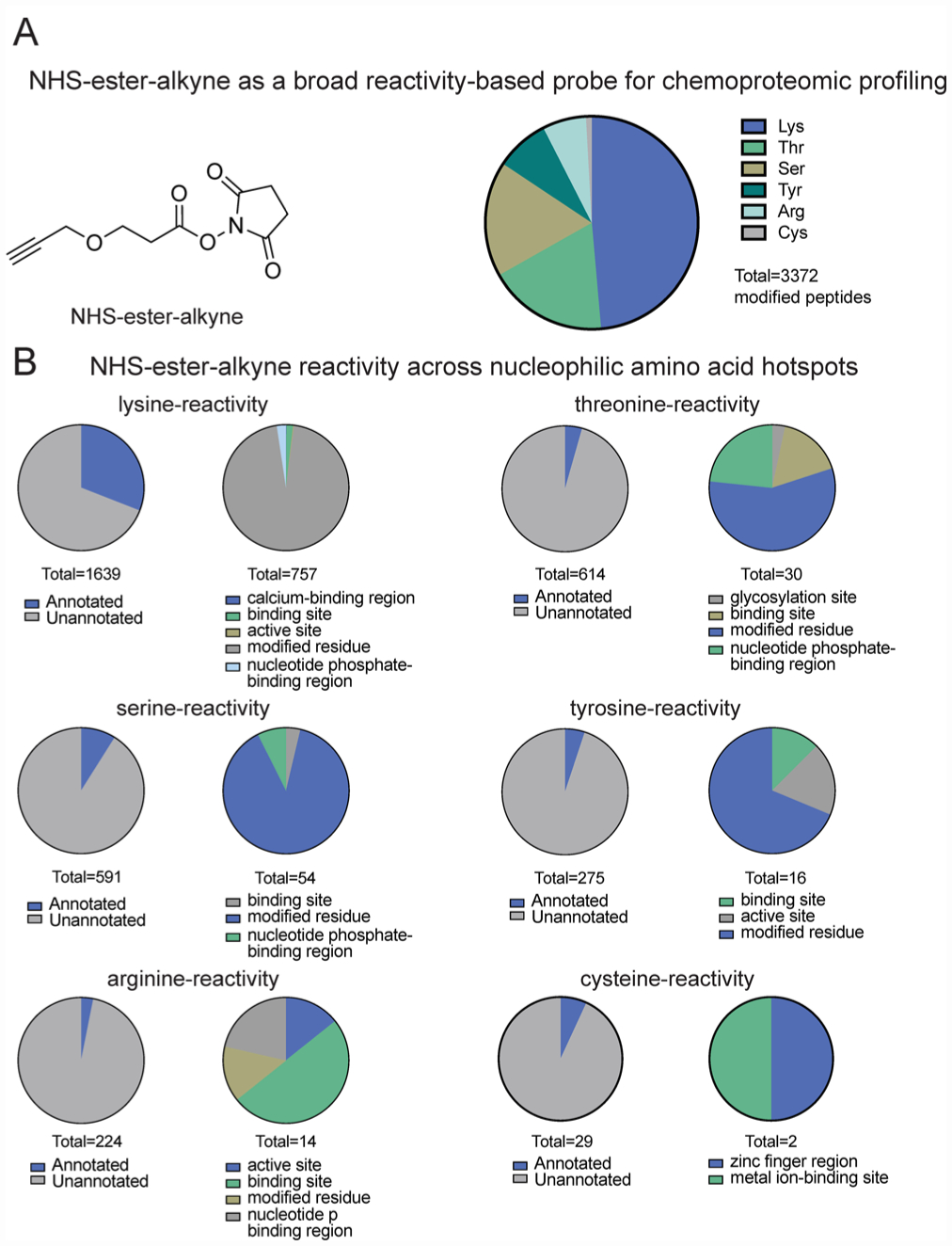
IsoTOP-ABPP of NHS-ester-alkyne reactivity in mouse liver proteome. (A) Structure of NHS-ester-alkyne (NHSyne) and distribution of probe-modified peptides in mouse liver proteome assessed by isoTOP-ABPP. Mouse liver proteomes were labeled with NHS-ester-alkyne (500 or 100 μM), followed by copper-catalyzed azide–alkyne cycloaddition (CuAAC) conjugation of a biotin-azide tag bearing an isotopically light (for 500 μM) or heavy (100 μM) mass tag to probe-labeled proteins. Probe-labeled proteins were subsequently avidin-enriched and digested with trypsin, and probe-modified tryptic peptides were isolated and eluted by TEV protease for subsequent LC-LC/MS/MS proteomic analysis. Raw data and ratiometric analysis of heavy to light peptides can be found in Figure S1 and Table S1. NHS-ester-alkyne predominantly reacted with lysines, showed significant reactivity with threonines and serines, and showed minor reactivity with tyrosines, arginines, and cysteines. (B) Breakdown of probe-labeled sites compared against annotated UniProt functional sites. While the majority of labeled residues are unannotated, the annotated sites were predominantly post-translationally modified or involved in ligand binding. Data shown in A and B are representative of probe-modified peptides found in two out of four biological replicates.
