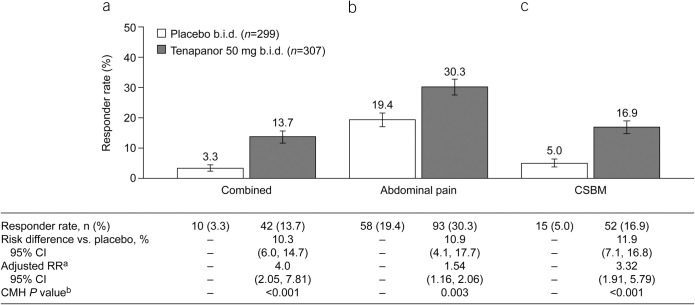
Figure 3.
Nine- of 12-week responder rates (ITT analysis set): proportions of patients with (a) combined response for ≥9 of 12 treatment weeks (key secondary efficacy variable), (b) abdominal pain response for ≥9 of 12 treatment weeks (key secondary efficacy variable), and (c) CSBM response for ≥9 of 12 treatment weeks (key secondary efficacy variable). aThe adjusted RR was based on the ratio of responder rates for tenapanor 50 mg b.i.d. vs placebo b.i.d., stratified by pooled investigator sites using the Mantel–Haenszel method. bThe CMH P value was based on a 1 degree of freedom test for association between treatment (tenapanor and placebo), stratified by pooled investigator sites. An abdominal pain response is defined as a decrease in average weekly worst abdominal pain of ≥30.0% from baseline. A CSBM response is defined as an increase of ≥1 CSBM/week from baseline. A combined response is defined as an abdominal pain response and CSBM response both occurring in the same week. b.i.d., twice daily; CI, confidence interval; CMH, Cochran–Mantel–Haenszel; CSBM, complete spontaneous bowel movement; ITT, intention-to-treat; RR, relative risk.