Figure 3. TAX1BP1 Mediates Aggregate Clearance.
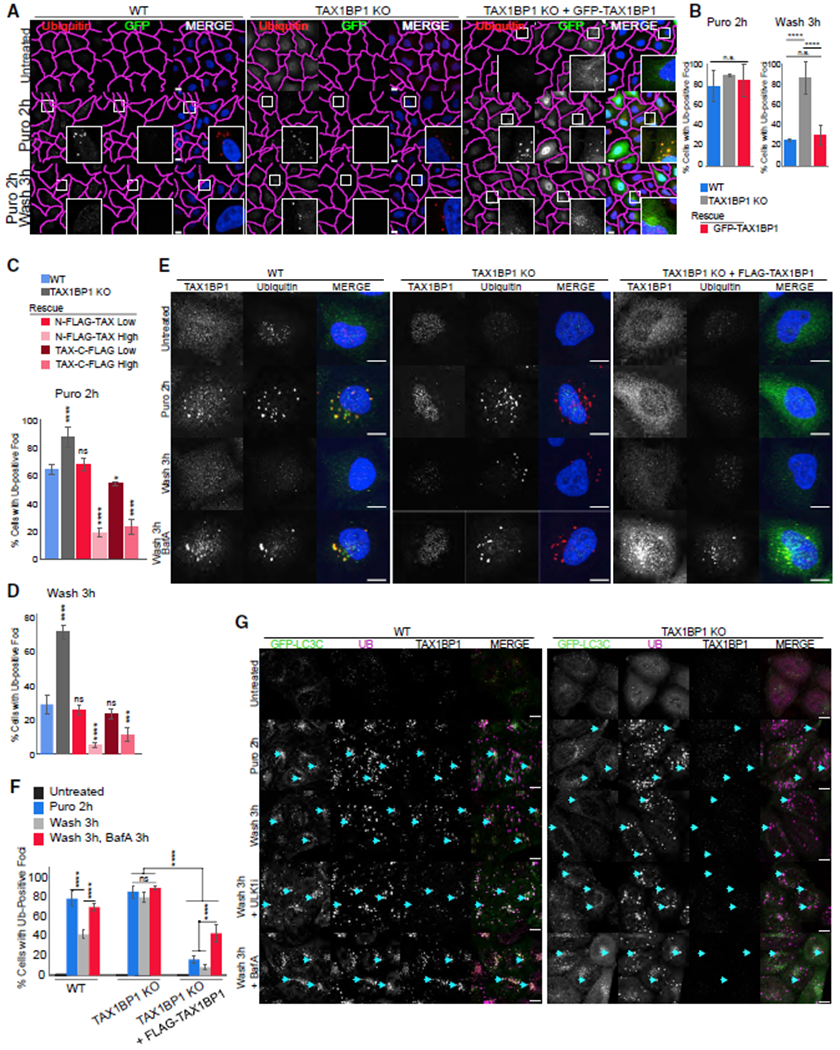
(A) WT, TAX1BP1 KO, and TAX1BP1 KO stably expressing GFP-TAX1BP1 were exposed to 5 μg/mL puromycin for 2 h, then fixed for imaging or washed and followed for a further 3 h in full media; scale bar represents 10 μm.
(B) Quantification of (A): percent of cells containing Ub-positive foci was assessed in ~200 cells per condition. Quantification displayed as mean ± SD from three independent experiments using one-way ANOVA test (****p < 0.0001) and Tukey’s post hoc test.
(C and D) Stably expressing TAX1BP1 rescue lines were created with N-FLAG or C-FLAG tag at high (H) or low (L) expression levels (see Figures S4A and S4B) and exposed to 5 μg/mL puromycin for 2 h, then either fixed for imaging (C) or washed and followed for 3 h in full media (D) and quantified as in (B). Quantification displayed as mean ± SD from three independent experiments using one-way ANOVA test (***p < 0.001, ****p < 0.0001) comparing all to WT and Tukey’s post hoc test.
(E) WT, TAX1BP1 KO, or TAX1BP1 KO + FLAG-TAX1BP1 (H) lines were exposed to 5 μg/mL puromycin in the presence or absence of 100 nM Bafilomycin A, then fixed for imaging or washed and followed for 3 h in full media or in media containing Bafilomycin A. Larger fields of view in Figure S4C.
(F) Quantification of (E): percent of cells containing Ub-positive foci was assessed in ~200 cells per condition in three independent experiments. Quantification displayed as mean ± SD from three independent experiments using two-way ANOVA test, (****p < 0.0001). All images are representative of at least three independent experiments.
(G) WT and TAX1BP1 KO cell lines stably expressing EGFP-LC3C were exposed to 5 μg/mL puromycin for 2 h in the presence or absence of 1 mM ULK1 inhibitor or 100 nM Bafilomycin A, after which cells were either fixed for imaging or washed and followed for 3 h in full media in the presence or absence of ULK1 inhibitor or Bafilomycin A; scale bar represents 10 μm.
