Abstract
Multiple system atrophy (MSA) is a neurodegenerative disease characterized by presence of α-synuclein-positive inclusions in the cytoplasm of oligodendrocytes. These glial cytoplasmic inclusions (GCIs) are considered an integral part of the pathogenesis of MSA, leading to demyelination and neuronal demise. What is most puzzling in the research fields of GCIs is the origin of α-synuclein aggregates in GCIs, since adult oligodendrocytes do not express high levels of α-synuclein. The most recent leading hypothesis is that GCIs form via transfer and accumulation of α-synuclein from neurons to oligodendrocytes. However, studies regarding this subject are limited due to the absence of proper human cell models, to demonstrate the entry and accumulation of neuronal α-synuclein in human oligodendrocytes. Here, we generated mature human oligodendrocytes that can take up neuron-derived α-synuclein and form GCI-like inclusions. Mature human oligodendrocytes are derived from neural stem cells via “oligosphere” formation and then into oligodendrocytes, treating the cells with the proper differentiation factors at each step. In the final cell preparations, oligodendrocytes consist of the majority population, while some astrocytes and unidentified stem cell-like cells were present as well. When these cells were exposed to α-synuclein proteins secreted from neuron-like human neuroblastoma cells, oligodendrocytes developed perinuclear inclusion bodies with α-synuclein immunoreactivity, resembling GCIs, while the stem cell-like cells showed α-synuclein-positive, scattered puncta in the cytoplasm. In conclusion, we have established a human oligodendrocyte model for the study of GCI formation, and the characterization and use of this model might pave the way for understanding the pathogenesis of MSA.
Keywords: Alpha-synuclein, Multiple system atrophy, Oligodendrocyte, Transmission, Neurodegeneration
INTRODUCTION
Multiple system atrophy (MSA) is a relatively rare, sporadic adult-onset neurodegenerative disorder. It is characterized by Parkinson-like motor symptoms, such as bradykinesia, rigidity, and postural instability, and non-motor symptoms of sleep and cognitive disorders, respiratory problems, and emotional/behavioral symptoms (Benrud-Larson et al., 2005; Jecmenica-Lukic et al., 2012; Lee et al., 2019). The main pathological characteristics of MSA are extended neuronal death and gliosis in several areas of the central nervous system. Filamentous glial cytoplasmic inclusions (GCIs) are predominantly found in oligodendrocytes and astrocytes (Papp et al., 1989). An excessive accumulation of α-synuclein in GCIs is accompanied by demyelination and degeneration of neurons and brain atrophy.
α-Synuclein is a neuronal protein that has been linked to several neurodegenerative diseases, such as Parkinson’s disease (PD), dementia with Lewy bodies, and MSA. It is found mostly in the cytoplasm but can be secreted from the cell and taken up by neighboring cells (Lee et al., 2005, 2014). Such “transmission” of α-synuclein is observed from neuron to neuron and glial cells, such as astrocytes and microglia (Desplats et al., 2009; Lee et al., 2010; Kim et al., 2013).
Mature human oligodendrocytes express little to no α-synuclein (Ozawa et al., 2001; Miller et al., 2005; Valera and Masliah, 2018). The oligodendrocytes of patients with MSA do not show an increased expression of α-synuclein. Consequently, it remains unclear how α-synuclein could accumulate in the GCIs of oligodendrocytes if these cells do not express a large amount of α-synuclein. One possible explanation is the “transmission” hypothesis of α-synuclein, which states the transfer of this protein from neurons to oligodendrocytes. The transfer of α-synuclein neurons to oligodendrocytes has been shown with the OLN-93 oligodendroglial cell line, where recombinant monomeric and oligomeric α-synuclein were detected inside the cells after treatment (Reyes et al., 2014). MSA oligodendrocytes might be more prone to accumulation of neuron-derived α-synuclein due to the dysfunctions of cellular clearance pathways. Such oligodendrocytes may lose their ability to support neurons and other cells and become toxic.
Animal models of MSA utilize the toxins injected in the brain or the transgenic expression of α-synuclein with oligodendrocyte-specific promoters [reviewed in Lee et al. (2019)]. However, only a few cellular models of MSA were created due to the difficulties in culturing oligodendrocytes. In this study, we optimized the culturing conditions for oligodendrocytes by differentiating human neural stem cells (hNSCs) into oligodendrocyte progenitor cells (OPCs) and then to oligodendrocytes. Differentiated oligodendrocytes were tested for the uptake of extracellular α-synuclein in the α-synuclein conditioned medium (CM) obtained from cultured SH-SY5Y human neuroblastoma cells. Results showed that α-synuclein accumulated as small round GCI-like inclusions near the nuclei, supporting the hypothesis that α-synuclein accumulation may arise from the “transfer” of α-synuclein from neighboring neurons into oligodendrocytes.
MATERIALS AND METHODS
Antibodies used
The primary antibodies used were as follows: α-synuclein monoclonal antibody (BD Biosciences, Franklin Lakes, NJ, USA), CNPase, GFAP, MBP, PLP antibodies (Abcam, Cambridge, UK), O4 antibody (Millipore, Darmstadt, Germany), and MAP2 antibody (Sigma, St. Louis, MO, USA).
SH-SY5Y cell culture and conditioned medium
The human neuroblastoma cell line, SH-SY5Y, was maintained and differentiated as described previously (Lee et al., 2004). The procedure for obtaining CM from α-synuclein is described elsewhere (Lee et al., 2010).
Maintenance of hNSC cells
StemPro® Neural Stem Cells (A15654, Thermo Fisher, Waltham, MA, USA), the human fetal brain-derived NSCs, were cultured according to the manufacturer’s protocol with some modifications.
The dishes were coated with CELLstart™ CTS™ (A10142-01, diluted 1:50, Thermo Fisher), Matrigel® hESC-Qualified Matrix (354277, diluted 1:100, Corning, NY, USA), and laminin (23017-015, 10 μg/mL, Thermo Fisher); diluted in DPBS with Ca2+/Mg2+ (A14040-117, Thermo Fisher), and incubated for 2 h at 37°C. Coated dishes were rinsed once with warm DMEM/F12 medium immediately before use.
The cells were maintained in a growth medium containing CTS™ KnockOut™ DMEM/F-12 (A1370801, Thermo Fisher) with StemPro™ Neural Supplement (A10508-01, 1:50, Thermo Fisher), human FGFβ (final 20 ng/mL, PeproTech, Cranbury, NJ, USA), human EGF (AF-100-15, final 40 ng/mL, PeproTech), GlutaMAX Supplement (35050-061, 1:100, Thermo Fisher), and penicillin-streptomycin (1:100) and incubated in a humidified 37°C, 5% CO2 incubator.
Differentiation of hNSCs into oligodendrocytes
Formation of oligospheres: StemPro hNSCs were detached from the culture dishes and counted. The medium was replaced with “OPC medium” (CTS™ KnockOut™ DMEM/F-12 with B-27™ supplement minus vit. A (12587-010, 1:50, Thermo Fisher), N2 supplement (17502-048, 1:100, Thermo Fisher), human FGFβ (100-188, final 20 ng/mL, PeproTech), PDGF-AA (CYT-341, 20 ng/mL, ProSpec, Rehovet, Israel), GlutaMAX Supplement (1:100), and penicillin-streptomycin (1:100)). After centrifugation, the cells were transferred to Nunclon Sphera™ dishes (4.5×106 cells/90mm dish). The medium was partially replaced with fresh OPC medium every 2-3 days for 7 days until oligospheres formed.
Dissociation of oligospheres into single OPCs: The oligodendrocyte-lineage cell culture dishes were coated with poly-L-ornithine (P3655, 40 μg/mL, Sigma) in sterilized deionized water at 37°C for 2 h. The dishes were rinsed twice with sterile water, and laminin (10 μg/mL) was added to ice-cold DPBS with Ca2+/Mg2+. The solution was incubated overnight at 37°C. The laminin solution was replaced with warm DMEM/F12 medium and kept at 37°C in an incubator until use.
Oligospheres were transferred to a 15-mL conical tube and centrifuged at 100×g for 3 min. After removing the medium, the cell pellet was resuspended 2-3 times with a warm accutase solution (A1110501, Thermo Fisher) and incubated at 37°C in a waterbath for 10 min with occasional tapping. The accutase was inactivated with the same volume of OPC medium, and the cells were spin down by centrifugation. The cell pellet was resuspended in fresh OPC medium and counted. The cells were transferred to an ornithine/lamin coated culture dish (up to 1×106 cells/35-mm dish). The cells were maintained in the OPC medium for 5 days. The medium was replaced every 2-3 days.
Differentiation of OPCs into oligodendrocytes: The cultured cells in OPC medium were detached and placed in a regular tissue culture dish and incubated for 30 min at 37°C. This procedure allows the removal of sticky cells, which are usually undifferentiated NSCs or differentiated astrocytes. The unattached cells were removed gently, and the process was repeated. The remaining cells were counted and placed on coverslips coated with ornithine/laminin. The cells were incubated in OPC medium for 2 days to allow cells to attach to the coverslips. The medium was replaced with “oligodendrocyte differentiation (OD) medium” (Neurobasal medium (21103-049, Thermo Fisher) with B-27™ supplement minus vit. A (1:50), 3,3′,5-Triiodo-L-thyronine sodium salt (T3, T2752, 30 ng/mL, Sigma), GlutaMAX Supplement (1:100), and penicillin-streptomycin (1:100)). The medium was replaced every 2-3 days for 14 days.
Differentiation of hNSCs into neurons
StemPro hNSCs were detached from the culture dishes and counted. The cells were placed on ornithine/laminin-coated coverslips (~3.5×105 cells) with the growth medium described above. After 2 days, the medium was replaced with a “neuron differentiation medium” (neurobasal medium with B-27™ supplement (17504-044, 1:50, Thermo Fisher), GlutaMAX Supplement (1:100), and penicillin-streptomycin (1:100)). The medium was changed every 2-3 days for 14 days.
Differentiation of hNSCs into astrocytes
The dishes for differentiation of astrocytes were coated with Matrigel (354277, 1:100, Corning) in ice-cold DPBS with Ca2+/Mg2+ and incubated for 2 h at 37°C. The Matrigel solution was replaced with warm DMEM/F12 medium and kept at 37°C in an incubator until use.
StemPro hNSCs were detached from the culture dishes and counted. The cells were plated at 2.5×104 cells/cm2. After 2 days, the medium was changed to “astrocyte differentiation medium” (DMEM with N2 supplement (1:100), fetal bovine serum (FBS, 1:100, Hyclone, South Logan, UT, USA), GlutaMAX Supplement (1:100), and penicillin-streptomycin (1:100)). The spent medium was changed every 3-4 days for up to 14 days.
Extracellular α-synuclein transfer into differentiated oligodendrocytes
OPCs were differentiated for 12-14 days, and the CM with α-synuclein was added at concentrations of 100 and 500 ng/mL.
Immunofluorescence cell staining
Cells grown on coverslips were fixed in 4% paraformaldehyde in PBS. Fixed cells were permeabilized with 0.1% Triton X-100 and washed with PBS before placed into the blocking solution (5% bovine serum albumin and 3% goat serum in PBS). The primary antibodies were diluted in blocking solution and added to the cells. After washing in PBS, the cells were incubated with the fluorescent dye Alexa488, Cy2, Rhodamine red-X, or Alexa647-conjugated secondary antibodies (Jackson Immunoresearch Laboratories, PA, USA) and then washed again in PBS. The nuclei were stained with TOPRO-3 dye (Thermo Fisher) or Hoechst 33342 (Sigma), and the coverslips were mounted on slides using the Antifade reagent (Thermo Fisher). The slides were observed under an Olympus FV1000 confocal laser-scanning microscope (Olympus, Tokyo, Japan) and Zeiss LSM 900 with Airyscan 2 (Zeiss, Oberkochen, Germany).
Statistical analysis
The data were analyzed, and the graph was drawn using the Prism 8 software (Graphpad Software Inc., San Diego, CA, USA).
RESULTS
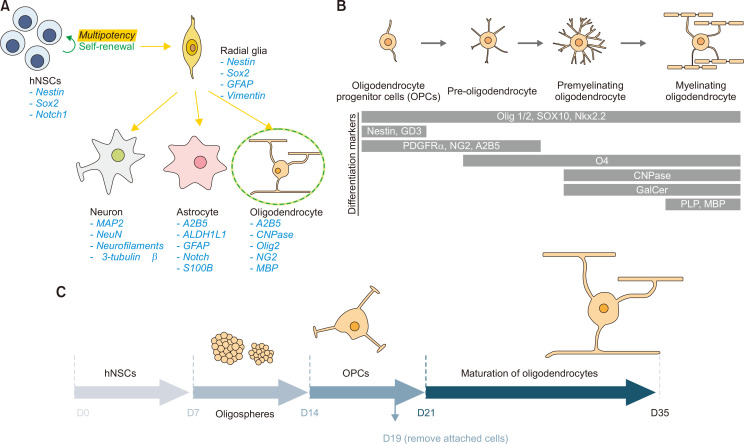
Neural stem cells (NSCs) are multipotent, self-renewing cells that can differentiate into neurons and glia of the central nervous system. These cells typically appear during the early embryonic development, but some cells persist in the adult brain throughout life. The first step of NSC differentiation is the formation of “radial glia,” the bipolar-shaped progenitor cells that could produce neurons, astrocytes, and oligodendrocytes. Various specific markers for each cell type are shown in Fig. 1A. Differentiation of NSCs into mature oligodendrocytes occurs in several stages where specific protein markers are observed (Fig. 1B). The first step of such transformation is to form oligodendrocyte precursor cells (OPCs) that can develop into oligodendrocytes and some neurons. OPCs become pre-oligodendrocytes that express oligodendrocyte-specific markers, such as O4. Further differentiation of the cells ultimately leads to mature oligodendrocytes that can myelinate neuronal axons (Fig. 1B).
Fig. 1.
Schematic representations of human NSC differentiation into various cell types. (A) Multipotent NSCs become bipolar radial glia, which then differentiate into neurons, astrocytes, and oligodendrocytes. Marker proteins for each cell type are shown. (B) Maturation of oligodendrocyte-lineage cells from OPCs to myelinating oligodendrocytes. Note the distinct cell markers of each step. (C) Optimized conditions for differentiating hNSCs into mature oligodendrocytes. Human NSCs are induced to form oligospheres, where these cells become OPCs. OPCs are further differentiated into oligodendrocytes.
In this study, human fetal brain-derived NSCs were utilized to optimize the conditions for oligodendrocyte differentiation. A schematic of the experimental protocol is shown in Fig. 1C. Highly proliferative and healthy hNSCs, grown as a monolayer, were detached and placed in hydrophobic surface dishes (Nunclon SpheraTM) to induce the formation of “oligospheres.” The media were replaced with those optimized for OPC growth. This step allows the enrichment of pre-OPCs and OPCs in three-dimensional space with close cell-cell contacts, which facilitates cellular transformation. The oligospheres are then dissociated and grown as a monolayer on coated plates to become highly proliferative OPCs. However, the cells at this point are heterogeneous in that they may contain less differentiated cells along the differentiation pathway. These mixtures of cells are detached again, and the sticky, non-oligodendrocyte lineage cells are removed by allowing them to settle on tissue culture plates for 30 min. Unattached OPCs floating in the medium are then transferred to coated coverslips and differentiated further to induce transformation into mature oligodendrocytes.
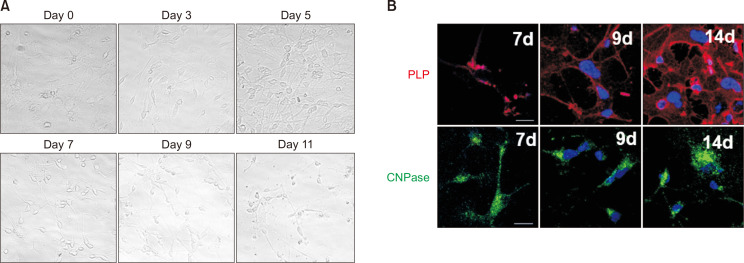
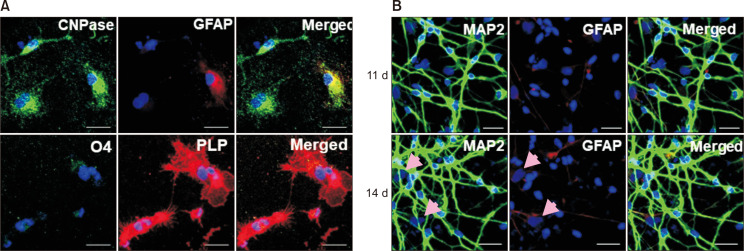
Fig. 2A shows the morphological changes from OPCs into oligodendrocytes. The fibroblast-like cell shapes of OPCs become more spherical with long, extended arms as days passed. The cells start to express oligodendrocyte markers, such as proteolipid protein (PLP) and 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase) at day 7, and these protein levels increase with time (Fig. 2B). By day 14, these differentiated cells expressed high levels of CNPase, PLP, and some O4, but only a few were GFAP positive (Fig. 3A). These results suggest that human NSCs can successfully differentiate into oligodendrocytes.
Fig. 2.
Differentiation of human NSCs into oligodendrocytes and neurons (A) Differentiated oligodendrocytes within 14 days express oligodendrocyte markers, CNPase, PLP, and O4. Only a few cells express GFAP, radial glia, and astrocyte marker. (B) The hNSCs differentiated into neurons, which expressed a high level of MAP2, a neuronal marker. Note that cells with large nuclei (pink arrows) do not stain for GFAP or MAP2 (scale bar: 20 μm).
Fig. 3.
Differentiation of human OPCs into oligodendrocytes. (A) Morphological changes in OPCs after differentiation in a time-dependent manner. (B) Differentiated cells express oligodendrocyte markers, PLP, and CNPase (scale bar: 20 μm).
The human NSCs were further tested for their differentiation into other cell types, such as neurons. More than 90% of cells differentiated into neurons expressed high levels of microtubule-associated protein 2 (MAP2), a neuronal marker protein (Fig. 3B).
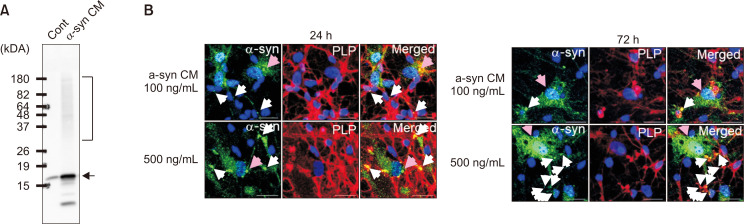
To test whether human oligodendrocytes could internalize extracellular α-synuclein, these cells were treated with CM containing secreted α-synuclein. SH-SY5Y CM contained monomeric and aggregated α-synuclein, as shown by Western blotting (Fig. 4A). α-Synuclein ELISA determined the α-synuclein concentration in the CM. Oligodendrocytes that differentiated for 12-14 days were treated with α-synuclein CM at final concentrations of 100 ng/mL or 500 ng/mL for 24 and 72 h (Fig. 4B). The results showed that α-synuclein was detected in oligodendrocyte cultures (white and pink arrows).
Fig. 4.
Extracellular α-synuclein uptake and inclusion formation by human oligodendrocytes. (A) Extracellular human α-synuclein in the CM of SH-SY5Y cells as shown by Western blotting (arrow: monomeric α-synuclein, bracket: aggregated α-synuclein). (B) Differentiated human oligodendrocytes were treated with α-synuclein CM at 100 ng/mL and 500 ng/mL concentrations for 24 and 72 h. PLP(+) cells show α-synuclein inclusion bodies (white arrows), but PLP(−) cells (pink arrows) have α-synuclein puncta scattered in the cytoplasm (scale bar: 20 μm).
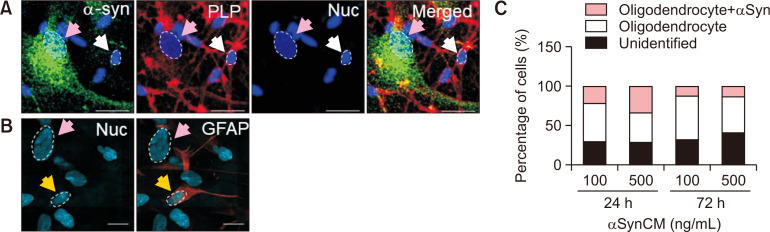
Interestingly, there seemed to be two different cell populations in these differentiated cells (Fig. 5A). One population had larger nuclei (pink arrows) that were negative for PLP. These cells stained remarkably well with the α-synuclein antibody and showed a punctate pattern scattered throughout the cytoplasm. The other population of cells, with smaller nuclei, stained positive for PLP. These PLP(+) differentiated oligodendrocytes (white arrows) also stained for α-synuclein inside the cells. However, α-synuclein in these cells resembled more perinuclear inclusion bodies than the scattered puncta observed in PLP(−) cells. The inclusion bodies resembled GCIs of MSA oligodendrocytes, which also have perinuclear inclusions that stained positive for α-synuclein (Arima et al., 1998).
Fig. 5.
Localization patterns of internalized α-synuclein in different cell populations. (A) The diameters of PLP(+) and PLP(−) cell nuclei and the α-synuclein staining patterns were compared. (B) Differentiated astrocytes were stained for GFAP (yellow arrows). Note that the cells with large nuclei (white arrow) are GFAP(−). (C) Quantitative analysis of oligodendrocytes with α-synuclein uptake (scale bar: 20 μm).
To verify whether the cells with large nuclei and scattered α-synuclein puncta (pink arrows, PLP(−)) were astrocytes or neurons, the NSCs were differentiated into astrocytes or neurons for 14 days. To our surprise, the cells with bigger nuclei (pink arrows) were GFAP(−) (Fig. 5B). Rather, GFAP(+) cells (yellow arrows) had smaller sized nuclei, about half the size of GFAP(−) cell nuclei. These cells with large nuclei did not stain with the neuronal marker MAP2 (Fig. 3B, pink arrows). Therefore, these results suggest that these PLP(−)/GFAP(−)/MAP2(−) cells with large nuclei may be undifferentiated NSCs or other cells (Fig. 1A). The α-synuclein uptake pattern differs in different types of NSC-derived cells. We are currently examining whether these PLP(−)/GFAP(−) cells are indeed undifferentiated cells by looking into markers such as Nestin and Sox2.
The ratio of PLP(+) cells (pink, gray bars) and unidentified PLP(−) cells (black bar) with large nuclei after differentiation showed that 55%-70% of cells were PLP(+) oligodendrocytes (Fig. 5C). Of these PLP(+) cells, 25%-30% of cells stained for α-synuclein. Together, these results suggest that human oligodendrocytes can take up extracellular α-synuclein and form perinuclear inclusion bodies. The uptake of α-synuclein leads to a pattern different from that of other cell types, including undifferentiated NSCs.
DISCUSSION
MSA is a rare neurodegenerative disorder with distinctive filamentous GCIs that are positive for α-synuclein in oligodendrocytes and astrocytes, concomitant with demyelination of axons and neuronal degeneration (Papp et al., 1989). In this study, we established an optimal condition for culturing human oligodendrocyte lineage cells that are differentiated from human NSCs. These differentiated human oligodendrocytes internalized secreted α-synuclein from neuronal cells and formed GCI-like inclusions in the periphery of cellular nuclei. Although most studies on α-synuclein uptake by oligodendrocytes have been performed with recombinant proteins, our study demonstrated that the neuron-derived extracellular α-synuclein could be taken up by human oligodendrocyte cells and form GCI-like inclusion bodies.
Several MSA cell and animal models have been reported to date. One of the approaches to establish these models was to overexpress α-synuclein directly in oligodendrocytes. α-Synuclein was overexpressed directly in the rat primary mixed culture, which showed some cytoplasmic α-synuclein inclusions in oligodendrocytes (Stefanova et al., 2005). α-Synuclein tg mice expressing human α-synuclein under oligodendrocyte-specific PLP, MBP, or CNP promoters showed pathological features of MSA with different degrees of motor and nonmotor symptoms and accumulation of α-synuclein aggregates in oligodendrocytes (Kahle et al., 2002; Shults et al., 2005; Yazawa et al., 2005). However, whether α-synuclein is expressed in mature, adult oligodendrocytes remains under debate. Many research groups failed to detect α-synuclein transcripts in these cells, although in one study, there was a three-fold increase in α-synuclein mRNA levels in postmortem MSA oligodendrocytes (Asi et al., 2014). Instead, recent studies support the hypothesis that α-synuclein could be transferred from neurons to other neighboring neurons and glia. This “transmission” theory was tested in several laboratories using MSA cell models. Recombinant α-synuclein aggregates were treated in OLN-93 cells, a rat oligodendrocytic cell line derived from the Wistar rat brain. This study showed that only aggregated α-synucleins were found inside the cells as small inclusions (Pukass and Richter-Landsberg, 2014; Reyes et al., 2014). Primary rat oligodendrocytes overexpressing α-synuclein transform internalized GCI-derived human α-synuclein aggregates into the high-seeding aggregate strain that can induce aggregation of other cells when taken up by other cells (Peng et al., 2018). These transmission cell models, however, have limitations as they are not human-originated cells. Furthermore, most of these studies used recombinant α-synuclein aggregates, which could differ structurally from cell-originated α-synuclein.
In our study, we established a method to differentiate human NSCs into mature oligodendrocytes as well as into neurons and astrocytes. The purity of differentiated oligodendrocytes was 50%-70%, varying depending on the culture conditions. Our study could be applied to NSCs derived from human iPSCs obtained from normal and MSA patients. The characterization of patient-derived oligodendrocytes would provide valuable insights into the pathogenic mechanisms of MSA. Our model can also be used for co-culture studies where differentiated neurons are cultured together with astrocytes and oligodendrocytes. Our MSA cell model would be a valuable tool for exploring the mechanism of “transmission” of α-synuclein from neurons into oligodendrocytes, hence the mechanism for GCI formation.
ACKNOWLEDGMENTS
This study was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government (NRF-2015R1D1A1A01059164, NRF2017R1A2B4003220, NRF-2016R1A5A2012284). This paper was written as part of Konkuk University’s research support program for its faculty on sabbatical leave in 2019.
REFERENCES
- Arima K., Ueda K., Sunohara N., Arakawa K., Hirai S., Nakamura M., Tonozuka-Uehara H., Kawai M. NACP/alpha-synuclein immunoreactivity in fibrillary components of neuronal and oligodendroglial cytoplasmic inclusions in the pontine nuclei in multiple system atrophy. Acta Neuropathol. (Berl.) 1998;96:439–444. doi: 10.1007/s004010050917. [DOI] [PubMed] [Google Scholar]
- Asi Y. T., Simpson J. E., Heath P. R., Wharton S. B., Lees A. J., Revesz T., Houlden H., Holton J. L. Alpha-synuclein mRNA expression in oligodendrocytes in MSA. Glia. 2014;62:964–970. doi: 10.1002/glia.22653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benrud-Larson L. M., Sandroni P., Schrag A., Low P. A. Depressive symptoms and life satisfaction in patients with multiple system atrophy. Mov. Disord. 2005;20:951–957. doi: 10.1002/mds.20450. [DOI] [PubMed] [Google Scholar]
- Desplats P., Lee H. J., Bae E. J., Patrick C., Rockenstein E., Crews L., Spencer B., Masliah E., Lee S. J. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jecmenica-Lukic M., Poewe W., Tolosa E., Wenning G. K. Premotor signs and symptoms of multiple system atrophy. Lancet Neurol. 2012;11:361–368. doi: 10.1016/S1474-4422(12)70022-4. [DOI] [PubMed] [Google Scholar]
- Kahle P. J., Neumann M., Ozmen L., Muller V., Jacobsen H., Spooren W., Fuss B., Mallon B., Macklin W. B., Fujiwara H., Hasegawa M., Iwatsubo T., Kretzschmar H. A., Haass C. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002;3:583–588. doi: 10.1093/embo-reports/kvf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Ho D. H., Suk J. E., You S., Michael S., Kang J., Joong Lee S., Masliah E., Hwang D., Lee H. J., Lee S. J. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Bae E. J., Lee S. J. Extracellular alpha--synuclein-a novel and crucial factor in Lewy body diseases. Nat. Rev. Neurol. 2014;10:92–98. doi: 10.1038/nrneurol.2013.275. [DOI] [PubMed] [Google Scholar]
- Lee H. J., Khoshaghideh F., Patel S., Lee S. J. Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway. J. Neurosci. 2004;24:1888–1896. doi: 10.1523/JNEUROSCI.3809-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Patel S., Lee S. J. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J. Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Ricarte D., Ortiz D., Lee S. J. Models of multiple system atrophy. Exp. Mol. Med. 2019;51:139. doi: 10.1038/s12276-019-0346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Suk J. E., Patrick C., Bae E. J., Cho J. H., Rho S., Hwang D., Masliah E., Lee S. J. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. W., Johnson J. M., Solano S. M., Hollingsworth Z. R., Standaert D. G., Young A. B. Absence of alpha-synuclein mRNA expression in normal and multiple system atrophy oligodendroglia. J. Neural Transm. 2005;112:1613–1624. doi: 10.1007/s00702-005-0378-1. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Okuizumi K., Ikeuchi T., Wakabayashi K., Takahashi H., Tsuji S. Analysis of the expression level of alpha-synuclein mRNA using postmortem brain samples from pathologically confirmed cases of multiple system atrophy. Acta Neuropathol. 2001;102:188–190. doi: 10.1007/s004010100367. [DOI] [PubMed] [Google Scholar]
- Papp M. I., Kahn J. E., Lantos P. L. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J. Neurol. Sci. 1989;94:79–100. doi: 10.1016/0022-510X(89)90219-0. [DOI] [PubMed] [Google Scholar]
- Peng C., Gathagan R. J., Covell D. J., Medellin C., Stieber A., Robinson J. L., Zhang B., Pitkin R. M., Olufemi M. F., Luk K. C., Trojanowski J. Q., Lee V. M. Cellular milieu imparts distinct pathological alpha-synuclein strains in alpha-synucleinopathies. Nature. 2018;557:558–563. doi: 10.1038/s41586-018-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukass K., Richter-Landsberg C. Oxidative stress promotes uptake, accumulation, and oligomerization of extracellular alpha-synuclein in oligodendrocytes. J. Mol. Neurosci. 2014;52:339–352. doi: 10.1007/s12031-013-0154-x. [DOI] [PubMed] [Google Scholar]
- Reyes J. F., Rey N. L., Bousset L., Melki R., Brundin P., Angot E. Alpha-synuclein transfers from neurons to oligodendrocytes. Glia. 2014;62:387–398. doi: 10.1002/glia.22611. [DOI] [PubMed] [Google Scholar]
- Shults C. W., Rockenstein E., Crews L., Adame A., Mante M., Larrea G., Hashimoto M., Song D., Iwatsubo T., Tsuboi K., Masliah E. Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. J. Neurosci. 2005;25:10689–10699. doi: 10.1523/JNEUROSCI.3527-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N., Reindl M., Neumann M., Haass C., Poewe W., Kahle P. J., Wenning G. K. Oxidative stress in transgenic mice with oligodendroglial alpha-synuclein overexpression replicates the characteristic neuropathology of multiple system atrophy. Am. J. Pathol. 2005;166:869–876. doi: 10.1016/S0002-9440(10)62307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E., Masliah E. The neuropathology of multiple system atrophy and its therapeutic implications. Auton. Neurosci. 2018;211:1–6. doi: 10.1016/j.autneu.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa I., Giasson B. I., Sasaki R., Zhang B., Joyce S., Uryu K., Trojanowski J. Q., Lee V. M. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron. 2005;45:847–859. doi: 10.1016/j.neuron.2005.01.032. [DOI] [PubMed] [Google Scholar]