Figure 1.
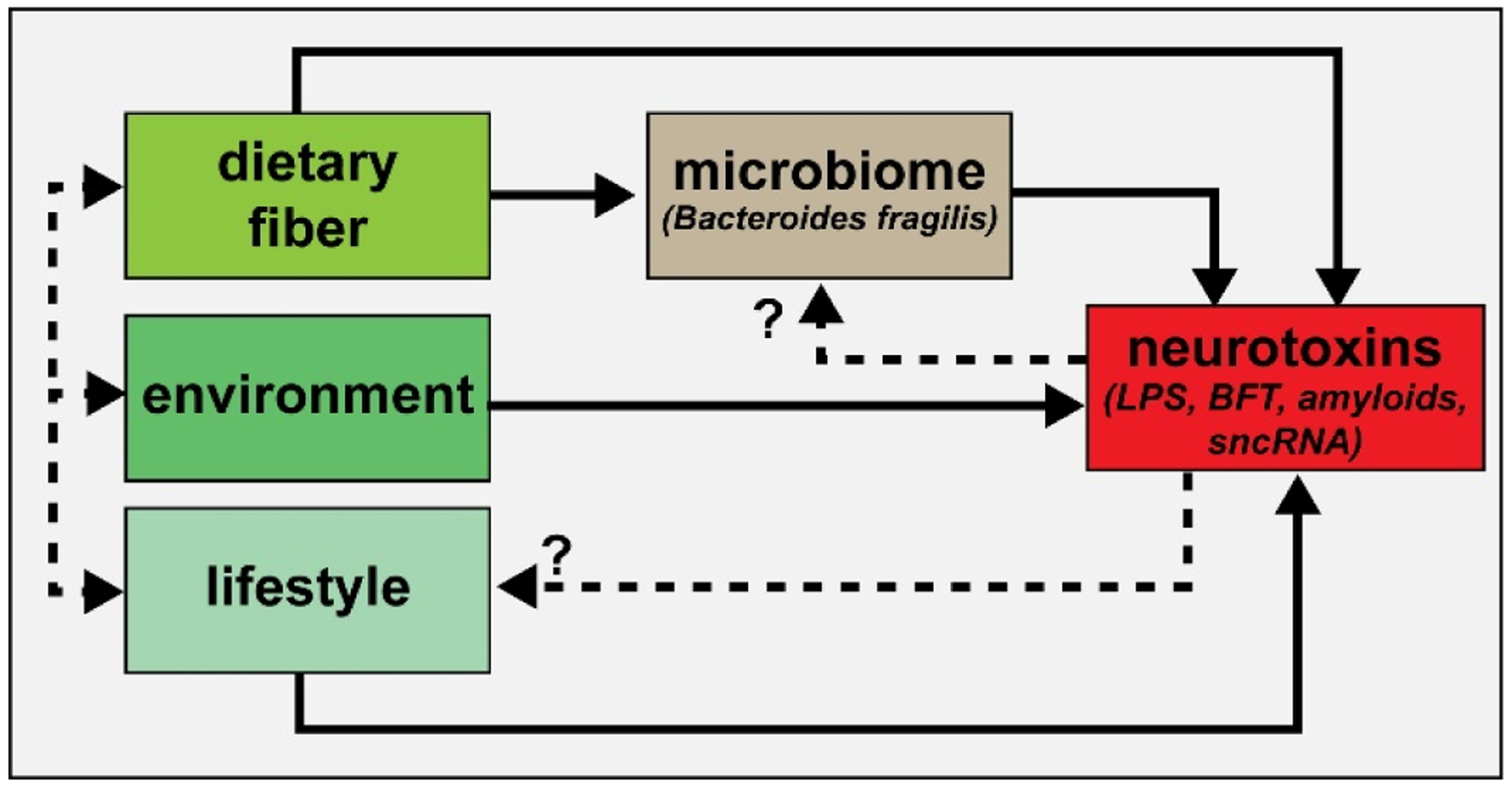
Highly simplified schematic of an integrated signaling system consisting of dietary-, environmental- and lifestyle-derived elements which provide neurotoxins – both neuro-inflammatory and neuro-pathogenic - to the brain and central nervous system (CNS). Dietary factors, such as both soluble and insoluble fiber from the diet are becoming increasingly appreciated as critical regulators of the abundance, speciation and health of microbial species in the microbiome [95–100]. In turn, abundant microbiome-resident Gram negative bacilli such as Bacteroides fragilis are known to secrete a formidable array of highly pro-inflammatory glycolipids, lipopolysaccharides (LPSs), lipooligosaccharides (LOS; smaller versions of LPS), barrier-disrupting enterotoxins such as BFT (fragilysin), bacterial amyloids and small non-coding RNAs (sncRNAs) that are known to affect the structure and function of biophysiological barriers such as the gastrointestinal (GI) tract barrier and blood-brain barrier (BBB) [16–21,43–49,94,100]. It is important to point out that B. fragilis (and its complex repertoire of neurotoxins) is just one of the many hundreds of thousands of bacterial subtypes resident in the GI-tract microbiome, and under normal physiological conditions there might be expected to be potentially generated an exceedingly variable and noxious mixture of multiple bacterial neurotoxins from many different microbial species. Dietary fiber intake, environment and lifestyle represent highly integrated components contributing to the maintenance of the microbiome and the potential for a life-long and continuous supply of neurotoxins (dashed black lines); the potential for contribution of neurotoxins via feedback mechanisms to microbiome abundance, complexity and speciation and lifestyle are currently suspected but are not well understood (dashed black lines with question marks)
