Abstract
Background
Dialysis patients have an increased bleeding risk as compared with the general population. However, there is limited information whether bleeding risks are different for patients treated with haemodialysis (HD) or peritoneal dialysis (PD). From a clinical point of view, this information could influence therapy choice. Therefore the aim of this study was to investigate the association between dialysis modality and bleeding risk.
Methods
Incident dialysis patients from the Netherlands Cooperative Study on the Adequacy of Dialysis were prospectively followed for major bleeding events over 3 years. Hazard ratios with 95% confidence intervals (CIs) were calculated for HD compared with PD using a time-dependent Cox regression analysis, with updates on dialysis modality.
Results
In total, 1745 patients started dialysis, of whom 1211 (69.4%) received HD and 534 (30.6%) PD. The bleeding rate was 60.8/1000 person-years for HD patients and 34.6/1000 person-years for PD patients. The time-dependent Cox regression analysis showed that after adjustment for age, sex, primary kidney disease, prior bleeding, cardiovascular disease, antiplatelet drug use, vitamin K antagonist use, erythropoietin use, arterial hypertension, residual glomerular filtratin rate, haemoglobin and albumin levels, bleeding risk for HD patients compared with PD increased 1.5-fold (95% CI 1.0–2.2).
Conclusions
In this large prospective cohort of incident dialysis patients, HD patients had an increased bleeding risk compared with PD patients. In particular, HD patients with a history of prior bleeding had an increased bleeding risk.
Keywords: bleeding, end-stage kidney disease, haemodialysis, peritoneal dialysis
KEY LEARNING POINTS
What is already known about this subject?
Dialysis patients have an increased bleeding risk as compared with the general population, however, information on whether bleeding risks are different for patients treated with haemodialysis (HD) or peritoneal dialysis (PD) is scarce.
Our study was conducted to provide prospective data regarding the difference in total bleeding risk between HD and PD patients.
What this study adds?
Our prospective study showed that in a group of 1211 HD and 534 PD patients, bleeding risk for HD patients compared with PD patients increased 1.5-fold.
In addition, a history of bleeding or the use of antiplatelet drugs or vitamin K antagonists led to highly increased bleeding risks for HD patients.
What impact this may have on practice or policy?
From a clinical perspective, these bleeding risks could be incorporated in the decision for a specific dialysis modality.
INTRODUCTION
For >30 years, end-stage kidney disease (ESKD) patients have been known to have an increased bleeding risk. Bleeding event rates for ESKD patients treated with haemodialysis (HD) or peritoneal dialysis (PD) range between 42 and 89/1000 person-years [1–5] compared with 0.5–0.9/1000 person-years in the general population [6–8]. The increased bleeding risk could be explained by anaemia (especially in the era before the introduction of erythropoietin), platelet dysfunction and impaired interaction between platelets and the vessel wall [9–11]. Furthermore, the high prevalence of antiplatelet and anticoagulant drug use could also play an important role [9, 11, 12].
There are limited data about differences in the bleeding risk of HD patients compared with PD patients. Most studies that have investigated bleeding risk in dialysis patients have focused on HD patients with atrial fibrillation. These studies showed a high bleeding risk in HD patients using vitamin K antagonists [13, 14]. Therefore there is doubt as to whether the benefit of vitamin K antagonists in preventing stroke outweighs the high bleeding risk in dialysis patients. Only four studies have compared the bleeding risk of patients on different dialysis modalities and showed that HD patients have a higher risk than PD patients for subdural haematomas and gastrointestinal bleeding [15–18]. Three of these studies were retrospective cohort studies conducted in Taiwan [15, 17, 18]. They showed that, compared with PD patients, HD patients have a 1.6-fold increased risk for subdural haematomas [15] and a 1.1- to 3.2-fold increased risk for gastrointestinal bleeding [17, 18]. However, prospective data regarding the difference in total bleeding risk between HD and PD patients are lacking.
From a clinical perspective, it is important to know whether HD compared with PD increases bleeding risk. There may be a preferred dialysis modality for specific subgroups of patients regarding bleeding risk. Therefore we investigated the association between dialysis modality and bleeding risk.
MATERIALS AND METHODS
Study population
The Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD), conducted in 38 dialysis centres, prospectively included ESKD patients who started dialysis treatment from 1997. Patients were ≥18 years of age and had no previous renal replacement therapy. Follow-up of patients was conducted until a bleeding event within 3 years of follow-up, death or censored in case of kidney transplantation, loss to follow-up or until December 2013. All patients provided written informed consent and local medical ethics committees approved the study.
Demographic and clinical data
Data on age, sex, dialysis modality and primary kidney disease were collected at the start of dialysis treatment. Primary kidney disease was classified according to the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) codes [19]. We grouped patients into four classes of primary kidney disease: diabetes mellitus, glomerulonephritis, renal vascular disease and other kidney diseases. Data on prior bleeding, cardiovascular disease, erythropoietin use and use of antithrombotic drugs (i.e. antiplatelet drugs or vitamin K antagonists) were also collected at the start of dialysis treatment. Prior bleeding was defined as a bleeding event leading to hospitalization and cardiovascular disease was defined as ischaemic heart disease (hospitalization for acute coronary syndrome or bypass surgery/percutaneous angioplasty), congestive heart failure or peripheral vascular disease. Blood pressure, haemoglobin, albumin, urea and creatinine were routinely measured in the dialysis centres at 3 months after the start of dialysis treatment. Blood pressure was measured before and after dialysis treatment over a 2-week period. The systolic and diastolic blood pressure values were both the average of up to six measurements. Arterial hypertension was defined as a systolic blood pressure ≥140 mmHg or a diastolic blood pressure ≥90 mmHg. Residual glomerular filtration rate (GFR) was calculated as the mean of creatinine and urea clearance, using creatinine and urea measurements in blood and 24-h urine collections (mL/min).
Bleeding
Bleeding was defined as an event leading to hospitalization or death within 3 years of follow-up. The following causes of death were classified as a result of bleeding: haemorrhagic pericarditis, gastrointestinal haemorrhage, haemorrhage from a peptic ulcer, haemorrhage from a vascular access or dialysis circuit, haemorrhage from a ruptured vascular aneurysm, haemorrhage from surgery and other haemorrhage (including cerebral and subdural haemorrhage) (ERA-EDTA codes 13, 23, 25–28, 71) [19].
Statistical analysis
Baseline characteristics are presented as percentages or median with interquartile range (IQR). Kaplan–Meier bleeding curves were generated for both dialysis modalities over 3 years of follow-up. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated for HD compared with PD using Cox proportional hazards analyses. Adjustment of HRs was first performed for baseline variables age, sex, primary kidney disease, prior bleeding, cardiovascular disease, antiplatelet drug use, vitamin K antagonist use and erythropoietin use. In addition, a second adjustment of HRs was performed in which arterial hypertension, residual GFR, haemoglobin and albumin levels were added to the other variables. Furthermore, a time-dependent Cox regression analysis, with updates on dialysis modality, was performed to account for the potential influence of changes in dialysis modality over time.
Multiple imputations were performed to account for missing data, using the fully conditional specification [20–23]. The imputation model contained all baseline characteristics including dialysis modality, bleeding outcome and mortality [21].
Interaction analyses were performed to identify patients with an increased bleeding risk. For these analyses, adjusted HRs of bleeding were calculated for HD patients with and without antithrombotic drug use, cardiovascular disease and prior bleeding compared with PD patients without antithrombotic drug use, cardiovascular disease and prior bleeding (reference group). The same reference group of PD patients was also used for calculation of the number needed to treat (NNT).
Statistical analyses were conducted with SPSS Statistics version 25 (IBM, Armonk, NY, USA).
RESULTS
Baseline characteristics
A total of 1745 patients were included, of whom 1211 patients (69.4%) started with HD and 534 patients (30.6%) with PD. Baseline characteristics are described in Table 1. HD patients, compared with PD, were older (66 versus 54 years), more often female (40% versus 35%), used antiplatelet drugs (26% versus 15%) and vitamin K antagonists (16% versus 5%) more often and had a slightly lower residual GFR (3 versus 4 mL/min). A small percentage of both HD and PD patients had a history of prior bleeding (7 and 4%, respectively).
Table 1.
Baseline characteristics
| HDa | PDb | |
|---|---|---|
| Characteristics | (n = 1211) | (n = 534) |
| Age (years), median (IQR) | 66 (54–73) | 54 (44–65) |
| Female sex, n (%) | 488 (40) | 187 (35) |
| Primary kidney disease, n (%) | ||
| Diabetes mellitus | 188 (16) | 93 (17) |
| Glomerulonephritis | 142 (12) | 96 (18) |
| Renal vascular disease | 260 (21) | 69 (13) |
| Other | 621 (51) | 276 (52) |
| Prior bleeding, n (%) | 83 (7) | 19 (4) |
| Cardiovascular disease, n (%) | 490 (40) | 134 (25) |
| Antiplatelet drug use, n (%) | 316 (26) | 80 (15) |
| Vitamin K antagonist use, n (%) | 195 (16) | 26 (5) |
| Erythropoietin use, n (%) | 896 (74) | 348 (65) |
| Arterial hypertension, n (%) | 750 (63) | 234 (45) |
| Residual GFR (mL/min), median (IQR) | 3 (1–5) | 4 (2–6) |
| Haemoglobin (mmol/L), median (IQR) | 6.7 (6.1–7.4) | 7.3 (6.7–8.0) |
| Albumin (g/L), median (IQR) | 37 (33–40) | 36 (33–40) |
n, number.
Missing in HD patients: prior bleeding, 12 (1.0%); arterial hypertension, 23 (1.9%); residual GFR, 269 (22.2%); haemoglobin, 20 (1.7%); albumin, 50 (4.1%).
Missing in PD patients: prior bleeding, 4 (0.7%); arterial hypertension, 11 (2.1%); residual GFR, 48 (9.0%); haemoglobin, 10 (1.9%); albumin, 18 (3.4%).
Bleeding events
Within 3 years of follow-up, 183 patients had a first bleeding event on dialysis after a median follow-up of 2.2 years (IQR 1.0–3.0). The bleeding rate was 52.3/1000 person-years. Of the 183 patients with bleeding events, 144 patients were treated with HD and 39 patients with PD at baseline. After 3 years, the cumulative bleeding incidence was 15.5% for HD patients and 9.7% for PD patients (Figure 1).
FIGURE 1.
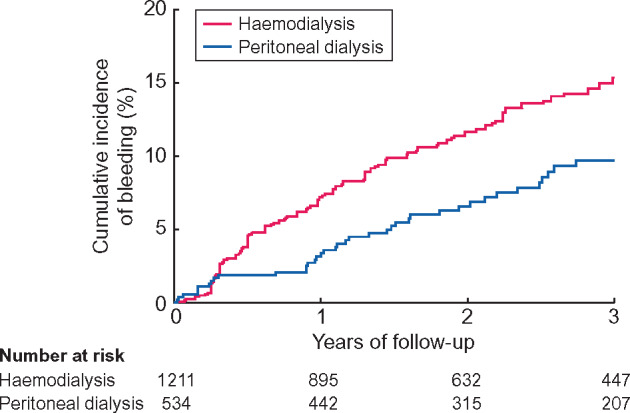
Cumulative bleeding incidence of dialysis patients.
HD patients had a bleeding rate of 60.8/1000 person-years and PD patients had a bleeding rate of 34.6/1000 person-years. The crude HR of bleeding was 1.7 (95% CI 1.2–2.5) in HD patients compared with PD patients. HD patients had a 1.5-fold (95% CI 1.0–2.1) increased bleeding risk after adjustment for age, sex, primary kidney disease, prior bleeding, cardiovascular disease, antiplatelet drug use, vitamin K antagonist use and erythropoietin use. Additional adjustment for arterial hypertension, residual GFR, haemoglobin and albumin levels resulted in a 1.4-fold (95% CI 1.0–2.1) increased bleeding risk (Table 2). The time-dependent Cox regression analysis showed a HR of 1.5 (95% CI 1.0–2.2) after adjustment for age, sex, primary kidney disease, prior bleeding, cardiovascular disease, antiplatelet drug use, vitamin K antagonist use, erythropoietin use, arterial hypertension, residual GFR, haemoglobin and albumin levels (Table 2).
Table 2.
HRs of bleeding for HD versus PD
| Time-dependent | ||||||
|---|---|---|---|---|---|---|
| Incidence rate/1000 | Crude | Adjusteda | Adjustedb | adjustedb | ||
| Dialysis modality | n | person-years | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| PD | 534 | 34.6 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| HD | 1211 | 60.8 | 1.7 (1.2–2.5) | 1.5 (1.0–2.1) | 1.4 (1.0–2.1) | 1.5 (1.0–2.2) |
Adjusted for age, sex, primary kidney disease, prior bleeding, cardiovascular disease, antiplatelet drug use, vitamin K antagonist use and erythropoietin use.
Adjusted for age, sex, primary kidney disease, prior bleeding, cardiovascular disease, antiplatelet drug use, vitamin K antagonist use, erythropoietin use, arterial hypertension, residual GFR, haemoglobin and albumin.
During the study, 13 patients died as a result of bleeding, of whom 12 were treated with HD and 1 with PD. Of the 12 fatal bleeding events in HD patients, 4 were due to haemorrhage from a ruptured vascular aneurysm, 3 due to gastrointestinal haemorrhage, 2 due to haemorrhage from surgery, 1 due to haemorrhage from the vascular access or dialysis circuit and 2 due to other haemorrhage. The fatal bleeding event in the PD patient was due to gastrointestinal haemorrhage. The fatal bleeding rate for HD patients was 5.1/1000 person-years and for PD patients was 0.9/1000 person-years.
Interaction analyses
First, stratification for antithrombotic drug use (i.e. antiplatelet drugs or vitamin K antagonists) was performed for which PD patients without antithrombotic drugs served as the reference group. The three groups for this analysis were HD patients without antithrombotic drugs, PD patients with antithrombotic drugs and HD patients with antithrombotic drugs. For HD patients without antithrombotic drugs, the time-dependent adjusted HR for bleeding was 1.7 (95% CI 1.1–2.7) compared with PD patients without antithrombotic drugs. For PD patients with antithrombotic drugs, the time-dependent adjusted HR was also 1.7 (95% CI 0.8–3.4). For HD patients with antithrombotic drugs, the time-dependent adjusted HR was 1.9 (95% CI 1.1–3.1) compared with the reference group. The NNT was 27 for HD patients with antithrombotic drugs (Table 3).
Table 3.
HRs of bleeding for HD versus PD stratified for antithrombotic drug use, cardiovascular disease and prior bleeding
| Dialysis modality | Stratification level | n | Incidence rate/ 1000 person-years | NNT | Crude HR (95% CI) | Adjusted HR (95% CI) | Time-dependent adjusted HR (95% CI) |
|---|---|---|---|---|---|---|---|
| Antithrombotic drug use | |||||||
| PD | No | 430 | 30.0 | Reference | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| HD | No | 712 | 56.6 | 38 | 1.9 (1.2–2.9) | 1.6a (1.0–2.6) | 1.7a (1.1–2.7) |
| PD | Yes | 104 | 56.4 | 38 | 1.8 (0.9–3.7) | 1.7a (0.8–3.5) | 1.7a (0.8–3.4) |
| HD | Yes | 499 | 67.3 | 27 | 2.2 (1.4–3.4) | 1.8a (1.1–3.0) | 1.9a (1.1–3.1) |
| Cardiovascular disease | |||||||
| PD | No | 400 | 30.9 | Reference | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| HD | No | 721 | 58.2 | 37 | 1.9 (1.2–2.9) | 1.6b (1.0–2.6) | 1.8b (1.1–2.9) |
| PD | Yes | 134 | 47.4 | 61 | 1.5 (0.8–3.0) | 1.3b (0.6–2.6) | 1.5b (0.8–2.9) |
| HD | Yes | 490 | 65.0 | 29 | 2.1 (1.3–3.2) | 1.4b (0.8–2.3) | 1.4b (0.8–2.5) |
| Prior bleeding | |||||||
| PD | No | 511 | 34.4 | Reference | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| HD | No | 1116 | 56.5 | 45 | 1.6 (1.1–2.4) | 1.4c (0.9–2.1) | 1.4c (1.0–2.1) |
| PD | Yes | 19 | 50.0 | 64 | 1.4 (0.3–6.0) | 1.3c (0.3–5.5) | 0.7c (0.1–5.3) |
| HD | Yes | 83 | 133.1 | 10 | 3.8 (2.2–6.6) | 2.8c (1.6–5.1) | 3.0c (1.7–5.3) |
Adjusted for age, sex, primary kidney disease, prior bleeding, cardiovascular disease, erythropoietin use, arterial hypertension, residual GFR, haemoglobin and albumin.
Adjusted for age, sex, primary kidney disease, prior bleeding, antiplatelet drug use, vitamin K antagonist use, erythropoietin use, arterial hypertension, residual GFR, haemoglobin and albumin.
Adjusted for age, sex, primary kidney disease, cardiovascular disease, antiplatelet drug use, vitamin K antagonist use, erythropoietin use, arterial hypertension, residual GFR, haemoglobin and albumin.
In addition, we analysed the two antithrombotic drugs separately. Vitamin K antagonists use led to a 1.8-fold (95% CI 1.1–3.1) increased (time-dependent adjusted) bleeding risk for HD patients compared with PD patients without vitamin K antagonists use. Antiplatelet drug use resulted in a time-dependent adjusted HR of 1.7 (95% CI 1.0–2.9) for bleeding in HD patients as compared with PD patients without antiplatelet drug use.
Second, stratification for cardiovascular disease was performed for which PD patients without cardiovascular disease served as the reference group. The three groups for this analysis were HD patients without cardiovascular disease, PD patients with cardiovascular disease and HD patients with cardiovascular disease. For HD patients without cardiovascular disease, the time-dependent adjusted HR for bleeding was 1.8 (95% CI 1.1–2.9) compared with PD patients without cardiovascular disease. For PD patients with cardiovascular disease, the time-dependent adjusted HR was 1.5 (95% CI 0.8–2.9). For HD patients with cardiovascular disease, the time-dependent adjusted HR was 1.4 (95% CI 0.8–2.5) compared with the reference group. The NNT was 29 for HD patients with cardiovascular disease (Table 3).
Third, stratification for prior bleeding was performed for which PD patients without prior bleeding served as the reference group. The three groups for this analysis were HD patients without prior bleeding, PD patients with prior bleeding and HD patients with prior bleeding. For HD patients without prior bleeding, the time-dependent adjusted HR for bleeding was 1.4 (95% CI 1.0–2.1) compared with PD patients without prior bleeding. For PD patients with prior bleeding, the time-dependent adjusted HR was 0.7 (95% CI 0.1–5.3). For HD patients with prior bleeding, the time-dependent adjusted HR was 3.0 (95% CI 1.7–5.3) compared with the reference group. The NNT was 10 for HD patients with prior bleeding (Table 3).
DISCUSSION
In this large prospective cohort of incident dialysis patients, both HD (60.8/1000 person-years) and PD patients (34.6/1000 person-years) had increased bleeding risks compared with the general population (0.5–0.9/1000 person-years) [6–8]. It is important to realize that the prevalence of antithrombotic drug use is higher in dialysis patients than in the general population [9, 11, 12]. The main finding of our study was that HD patients had a 1.5-fold increased bleeding risk compared with PD patients after adjustment for confounders. In addition, HD patients had highly increased bleeding risks when they used antithrombotic drugs or had a history of bleeding, which resulted in low NNTs (27 and 10, respectively). The importance of previous bleeding in an increased risk of new bleeding events is consistent with previous studies, which showed that this was the most important risk factor [3, 24].
This is the first prospective study comparing the bleeding risk of HD and PD patients taking into account all bleeding events. So far the bleeding risk has only been investigated in American and Taiwanese cohorts, which also showed an increased bleeding risk for HD patients compared with PD patients [15–18]. However, unlike our study, the studies in these cohorts all focused on a single bleeding source, namely gastrointestinal or subdural. In the American cohort described by Wasse et al. [16], 698 upper gastrointestinal bleeding cases among dialysis patients were investigated. The adjusted relative risk (RR) for a first upper gastrointestinal bleeding was non-significantly lower for PD patients compared with HD patients [RR 0.88 (95% CI 0.72–1.07)] . In the Taiwanese cohort, three retrospective studies were conducted [15, 17, 18]. First, the study by Wang et al. [15] described subdural haematomas among 10 136 HD and 10 136 PD patients. The adjusted HR of a subdural haematoma was significantly higher for HD patients compared with PD patients [HR 1.62 (95% CI 1.17–2.33)]. Second, the study of Lee et al. [17] described gastrointestinal bleeding events combined with diverticula among 8955 HD and 1791 PD patients. With 1417 events (1274 in HD patients and 143 in PD patients), the risk was significantly lower in PD patients compared with HD patients [HR 0.78 (95% CI 0.64–0.96)]. Finally, the study by Huang et al. [18] described peptic ulcer bleeding events among 2328 HD and 2239 PD patients. The adjusted risk for peptic ulcer bleeding compared with a control group of patients without kidney disease was lower for PD patients [HR 3.71 (95% CI 2.00–6.87)] than for HD patients [HR 11.96 (95% CI 7.04–20.31)] [18].
A possible explanation for the increased bleeding risk of HD patients could be the use of low molecular weight heparin during HD sessions, which is necessary to prevent clotting in the extracorporeal system [25, 26]. In particular, the combination of high heparin dosages during HD sessions and vitamin K antagonist use could have led to an increased bleeding risk. There is recent debate about whether the benefit (i.e. stroke reduction) of vitamin K antagonists in HD patients outweighs the bleeding risk [27]. In PD patients, the stroke and bleeding risks associated with vitamin K antagonists could be different. A previous study showed that warfarin reduced the incidence of stroke without increasing the risk of intracranial haemorrhage in PD patients [28]. Also, the increased bleeding risk in HD patients could result from intermittent puncture of the vascular access with needles. Unfortunately, data regarding the bleeding risk specifically related to the vascular access were lacking in our study. Another explanation for the increased bleeding risk of HD patients could be that those patients are less vital than PD patients. Although we have adjusted for many confounders, residual confounding could not be excluded.
To our knowledge, this is the first large prospective cohort study comparing overall bleeding risk of HD and PD patients. While prior studies primarily focused on gastrointestinal bleeding sources, our study also incorporated non-gastrointestinal bleeding sources in all dialysis patients. Furthermore, the accuracy of the recorded data is high, since nurses and nephrologists who treated these dialysis patients recorded the bleeding events. Our study has several limitations. First, data were collected between 1997 and 2013, a period when strategies regarding the use of antithrombotic drugs differed from current practice. However, we believe that the results are still relevant for dialysis patients today. Second, bleeding was defined as death due to bleeding or bleeding requiring hospitalization but was not validated or defined by the bleeding criteria of the International Society on Thrombosis and Haemostasis [29]. However, we think that our definition of bleeding incorporates important clinical endpoints. Third, data about the presence of atrial fibrillation or the use of heparin were missing. Another limitation of our study is the possibility of detection bias. Bleeding could be more often detected in HD patients than in PD patients since most HD patients visit a dialysis centre three times a week and therefore have more contact with healthcare professionals. However, we think that the detection bias is limited since we used bleeding requiring hospitalization as an outcome. In case of such major bleeding, we believe that PD patients will also seek contact with healthcare professionals. Finally, theoretically it could be possible that confounding by indication occurred, as patients were not randomized between HD or PD. Although the bleeding risk is usually not taken into account when choosing a dialysis modality, we have corrected for multiple confounders in our analysis. Since randomized controlled trials comparing bleeding rates of HD and PD patients will probably never be conducted, clinicians should make decisions together with their patients based on observational studies.
In conclusion, HD patients have a 1.5-fold increased bleeding risk compared with PD patients. An important subgroup is patients with previous bleeding problems. These patients may have an even higher bleeding risk with HD. ESKD patients should receive information about all treatments and subsequently make shared decisions with their nephrologists [30]. Ideally, the bleeding risk for a patient with a specific (bleeding) history could be incorporated in this decision since bleeding can potentially lead to hospitalization or death.
ACKNOWLEDGEMENTS
We thank the investigators and study nurses of the dialysis centres for participating in the Netherlands Cooperative Study on the Adequacy of Dialysis and for collection of the data. We also want to thank the data managers for management of the data.
FUNDING
The Netherlands Cooperative Study on the Adequacy of Dialysis was funded by an unrestricted grant (E93.018) from the Dutch Kidney Foundation. The Dutch Kidney Foundation was not involved in the collection, interpretation and analysis of the data or in decisions on the writing and submission of this report for publication.
AUTHORS’ CONTRIBUTIONS
G.O. and F.D. designed the study. G.O. and M.D. collected the data. G.O. analysed the data. A.E.S. and G.O. made the tables and figure and drafted and revised the article. All authors critically edited the manuscript and approved the final version.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest to declare. The results in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Olesen JB, Lip GY, Kamper AL. et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 2012; 367: 625–635 [DOI] [PubMed] [Google Scholar]
- 2. Yang JY, Lee TC, Montez-Rath ME. et al. Trends in acute nonvariceal upper gastrointestinal bleeding in dialysis patients. J Am Soc Nephrol 2012; 23: 495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sood MM, Larkina M, Thumma JR. et al. Major bleeding events and risk stratification of antithrombotic agents in hemodialysis: results from the DOPPS. Kidney Int 2013; 84: 600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Molnar AO, Bota SE, Garg AX. et al. The risk of major hemorrhage with CKD. J Am Soc Nephrol 2016; 27: 2825–2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong CX, Odutayo A, Emdin CA. et al. Meta-analysis of anticoagulation use, stroke, thromboembolism, bleeding, and mortality in patients with atrial fibrillation on dialysis. Am J Cardiol 2016; 117: 1934–1941 [DOI] [PubMed] [Google Scholar]
- 6. Vanleerdam M, Vreeburg E, Rauws E. et al. Acute upper GI bleeding: did anything change? Am J Gastroenterol 2003; 98: 1494–1499 [DOI] [PubMed] [Google Scholar]
- 7. Hernández-Díaz S, García Rodríguez LA.. Incidence of serious upper gastrointestinal bleeding/perforation in the general population: review of epidemiologic studies. J Clin Epidemiol 2002; 55: 157–163 [DOI] [PubMed] [Google Scholar]
- 8. Hreinsson JP, Kalaitzakis E, Gudmundsson S. et al. Upper gastrointestinal bleeding: incidence, etiology and outcomes in a population-based setting. Scand J Gastroenterol 2013; 48: 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pavord S, Myers B.. Bleeding and thrombotic complications of kidney disease. Blood Rev 2011; 25: 271–278 [DOI] [PubMed] [Google Scholar]
- 10. Remuzzi G. Bleeding in renal failure. Lancet 1988; 331: 1205–1208 [DOI] [PubMed] [Google Scholar]
- 11. Lutz J, Menke J, Sollinger D. et al. Haemostasis in chronic kidney disease. Nephrol Dial Transplant 2014; 29: 29–40 [DOI] [PubMed] [Google Scholar]
- 12. Reinecke H, Brand E, Mesters R. et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 2009; 20: 705–711 [DOI] [PubMed] [Google Scholar]
- 13. Winkelmayer WC, Liu J, Setoguchi S. et al. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol 2011; 6: 2662–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shah M, Tsadok MA, Jackevicius CA. et al. Warfarin use and risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation 2014; 129: 1196–1203 [DOI] [PubMed] [Google Scholar]
- 15. Wang IK, Cheng YK, Lin CL. et al. Comparison of subdural hematoma risk between hemodialysis and peritoneal dialysis patients with ESRD. Clin J Am Soc Nephrol 2015; 10: 994–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wasse H, Gillen DL, Ball AM. et al. Risk factors for upper gastrointestinal bleeding among end-stage renal disease patients. Kidney Int 2003; 64: 1455–1461 [DOI] [PubMed] [Google Scholar]
- 17. Lee YC, Hung SY, Wang HH. et al. Different risk of common gastrointestinal disease between groups undergoing hemodialysis or peritoneal dialysis or with non-end stage renal disease: a nationwide population-based cohort study. Medicine (Baltimore )2015; 94: e1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang KW, Leu HB, Luo JC. et al. Different peptic ulcer bleeding risk in chronic kidney disease and end-stage renal disease patients receiving different dialysis. Dig Dis Sci 2014; 59: 807–813 [DOI] [PubMed] [Google Scholar]
- 19. van Dijk PC, Jager KJ, de Charro F. et al. Renal replacement therapy in Europe: the results of a collaborative effort by the ERA-EDTA registry and six national or regional registries. Nephrol Dial Transplant 2001; 16: 1120–1129 [DOI] [PubMed] [Google Scholar]
- 20. Moons KG, Donders RA, Stijnen T. et al. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol 2006; 59: 1092–1101 [DOI] [PubMed] [Google Scholar]
- 21. Marshall A, Altman DG, Holder RL. et al. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol 2009; 9: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kenward MG, Carpenter J.. Multiple imputation: current perspectives. Stat Methods Med Res 2007; 16: 199–218 [DOI] [PubMed] [Google Scholar]
- 23. de Goeij MC, van Diepen M, Jager KJ. et al. Multiple imputation: dealing with missing data. Nephrol Dial Transplant 2013; 28: 2415–2420 [DOI] [PubMed] [Google Scholar]
- 24. Genovesi S, Rossi E, Gallieni M. et al. Warfarin use, mortality, bleeding and stroke in haemodialysis patients with atrial fibrillation. Nephrol Dial Transplant 2015; 30: 491–498 [DOI] [PubMed] [Google Scholar]
- 25. Bartels PC, Schoorl M, Schoorl M. et al. Activation of coagulation during treatment with haemodialysis. Scand J Clin Lab Invest 2000; 60: 283–290 [DOI] [PubMed] [Google Scholar]
- 26. Janssen MJFM, van der Meulen J.. The bleeding risk in chronic haemodialysis: preventive strategies in high-risk patients. Netherlands J Med 1996; 48: 198–207 [DOI] [PubMed] [Google Scholar]
- 27. Van Der Meersch H, De Bacquer D, De Vriese AS.. Vitamin K antagonists for stroke prevention in hemodialysis patients with atrial fibrillation: a systematic review and meta-analysis. Am Heart J 2017; 184: 37–46 [DOI] [PubMed] [Google Scholar]
- 28. Chan PH, Huang D, Yip PS. et al. Ischaemic stroke in patients with atrial fibrillation with chronic kidney disease undergoing peritoneal dialysis. Europace 2016; 18: 651– 671 [DOI] [PubMed] [Google Scholar]
- 29. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 692–694 [DOI] [PubMed] [Google Scholar]
- 30. Moss AH. Revised dialysis clinical practice guideline promotes more informed decision-making. Clin J Am Soc Nephrol 2010; 5: 2380–2383 [DOI] [PubMed] [Google Scholar]


