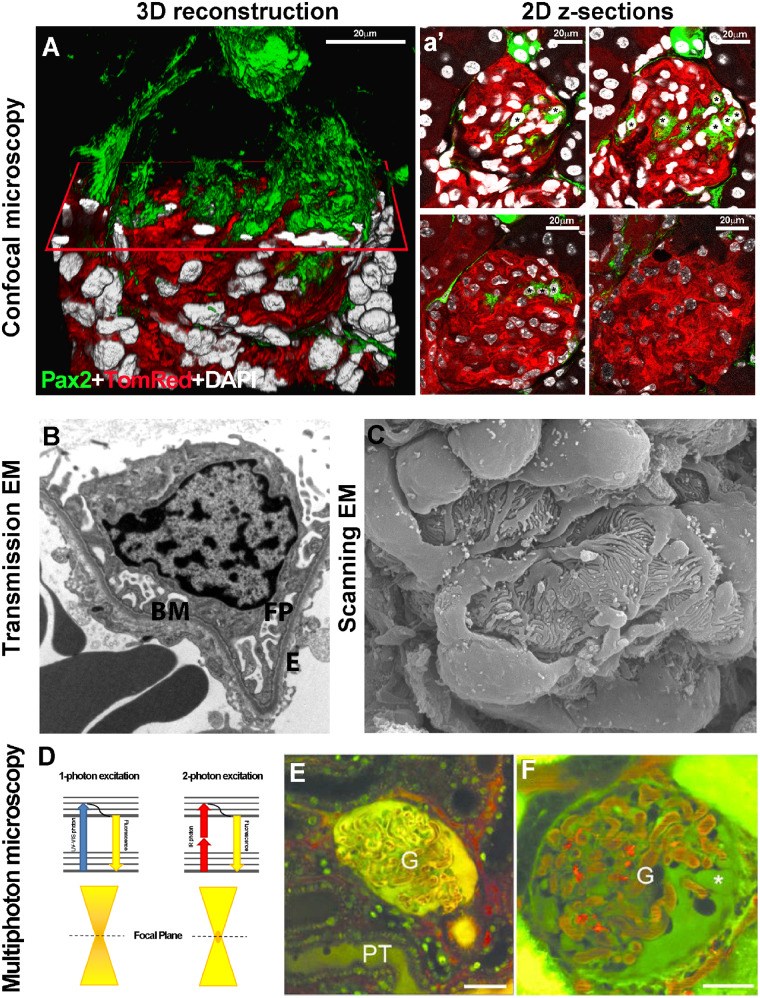
FIGURE 1.
(A) 3D reconstruction of a glomerulus from a 50-μm-thick kidney section, showing de novo podocyte regeneration in a transgenic mouse model of adriamycin-induced nephropathy. The fluorescent reporter GFP (green) labels Pax2+ renal progenitors, whereas Tomato Red labels all other cell types. An x-plane (red) has been added to virtually dissect the glomerulus in two parts. In the upper part, only green signal is shown; in the lower part, all three colours are shown. (a’) Representative 2D z-section stacks of the 3D reconstruction shown in (A). Asterisks indicate the different number of Pax2+ progenitor-derived podocytes counted inside the glomerular tuft in each 2D image. The z-stack analysis used to perform the 3D reconstruction of the glomerulus showed that the detection of regenerated podocytes was highly dependent on which section was used for 2D analysis and its thickness. (Adapted from Romoli et al. [6]. This article is licenced under a Creative Common Attribution 4.0 International License. http://creativecommons.org/licenses/by/4.0/.) (B) Representative image of a glomerular capillary loop as seen with TEM. BM, basement membrane; FP, foot processes; E, endothelial cell (TEM ×5000) (Adapted from Liapis [10] by permission of the publisher Taylor & Francis, http://www.tandfonline.com.) (C) SEM of a healthy glomerulus reveals octopus-like podocytes wrapping the capillary loops with interdigitating foot processes capillary loops (SEM ×140 000). (Adapted from Liapis [10] by permission of the publisher Taylor & Francis, http://www.tandfonline.com.) (D) The Jablonski diagram shows a comparison between one- and two-photon absorption. Conventional one-photon excitation uses ultraviolet or visible light to excite fluorescent molecules, whereas two-photon excitation depends on the simultaneous absorption of two photons with double λ (infrared light). Compared with one-photon excitation, the fluorescence excitation (orange) of two-photon excitation is spatially restricted to a small point within the focal plane, because the fluorescence excitation occurs only where the density of illuminating photons is highest. This reduces tissue photobleaching compared with the confocal approach. Representative MPM images of glomeruli (G) in vivo in (E) control or (F) PAN-treated Munich–Wistar–Fromter rat kidneys. The freely filtered dye, Lucifer Yellow, injected intravenously, labelled the Bowman’s space and early proximal tubule (PT) and allowed negative labelling of podocytes and parietal cells (which do not take up the dye). Cell nuclei were labelled using Hoechst33342 (green). The intravascular space (plasma) was labelled red by 70-kDa dextran rhodamine B injected intravenously. Unlike a normal glomerular structure (E), podocytes develop numerous pseudocysts (asterisk) after PAN treatment (F). Scale bars: 20 μm. (Adapted from Peti-Peterdi [17] with permission from Elsevier.)