Abstract
Background
Entada phaseoloides is a well-known medicinal plant traditionally used in Ayurvedic medicine for centuries.
Objective
To evaluate the anti-stress activity of seeds of E. phaseoloides in endoplasmic reticulum stress during chronic restrain stress in mice, based on our preliminary screening.
Materials and Methods
Mice (n = 6/group) were restrained daily for 6 h in 50 ml polystyrene tubes for 28 days. Methanolic extract of E. phaseoloides (MEEP) (100 and 200 mg/kg, p.o.) and standard drug, imipramine (10 mg/kg i.p.) were administered daily 45 min prior to restrain from day 22–28. Then, forced swim test (FST) was performed to assess despair behavior. Lipid peroxidation (LPO) and antioxidant enzymes Reduced glutathione (GSH), Superoxide dismutase (SOD) were measured in the hippocampus of mice. 78 kDa Glucose-regulated Protein, 94 kDa Glucose-regulated Protein, C/EBP homologous protein, Caspase-12 expression were quantified by Real Time PCR.
Results
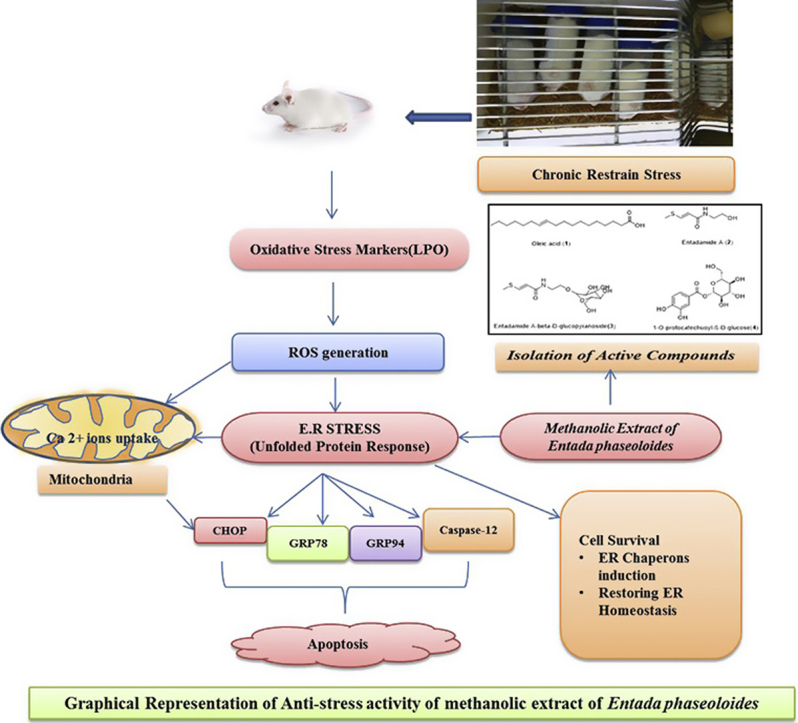
MEEP significantly reduced the immobility time in FST (P < 0.001). Significant reduction of LPO (P < 0.05) level and restored antioxidant enzymes viz. GSH (P < 0.001) and SOD towards vehicle control group were observed. Down-regulation of genes GRP 78, GRP 94 (P < 0.001), CHOP and Caspase-12 (P < 0.001) as compared to the chronic restrain stress group was evident, which were upregulated following treatment. Isolation of the active components of the seeds revealed the presence of Oleic acid (1), Entadamide A (2), Entadamide A-beta-d-glucopyranoside (3) and 1-O-protocatechuoyl-β-d-glucose.
Conclusion
MEEP altered endoplasmic reticulum stress in chronic restrain stressed mice; however, as an antidepressant it showed a weaker response.
Keywords: Antioxidant enzymes, Entada phaseoloides, Endoplasmic reticulum stress, Imipramine, Oxidative stress
Graphical abstract
1. Introduction
Depression is a prevalent psychiatric dysfunction that is manifested by various symptoms including depressed mood, loss of interest, feelings of guilt, disturbance of sleep and appetite, low power and poor centralization, interfering with normal function in day to day life [1]. According to the World Health Organization (WHO), depression will become the second factor contributing to the disability of disease in 2020 [2]. One of the most important factors for the depressive-like behaviour leading to various disorders is oxidative stress [3].
The Endoplasmic Reticulum (ER) organelle contained in the eukaryotic cell serves as the main site for synthesis of steroids, cholesterol and other lipids and also facilitates protein folding and maturation. Homeostasis in the ER is regulated through a synchronized adaptive programme, known as unfolded protein response (UPR) [4], [5], [6]. Disturbances in ER homeostasis affect the protein folding and can lead to the ER stress and elicit apoptotic signals. ER stress has been implicated in the pathogenesis of diabetes, ischemic and neurodegenerative disorders like Parkinson's and Alzheimer's disease [7].
Chronic restrain stress can lead to depressive-like behavior by causing cell death, neuro-inflammation and neurotoxicity, involves multiple factors such as Pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, IFN-γ), Ca2+ ion release and generation of Reactive Oxygen Species. These events cause subsequent involvement of the activation of ER-resident caspase-12, caspase-3 [8], [9].
African dream herb, Entada rheedii, shares its medicinal properties with Entada phaseoloides [10]. However scientific validation is not reported so far. The plant produces, varying quantities, saponins, fatty oils and other potentially psychoactive alkaloids [10]. Moreover, this herb is believed to have magico-religious beliefs [11]. This forms the basis of our study.
E. phaseoloides (Linn.) Merr. (Family: Fabaceae) is a well-known traditional medicinal plant distributed throughout the sub-Himalayan tract, in the monsoon forest of Western and Eastern Ghats and also in Andaman & Nicobar islands. The use of E. phaseoloides as folk lore/traditional remedy for various disease conditions and multifarious medicinal properties has been reported from time to time. Almost all of the parts this plant is used in indigenous systems of medicine, the people in tropical and sub-tropical regions of countries made preparations for the treatment of a wide variety of illnesses, including haemorrhoids, stomach ache, toothache, spasm, gastritis, and lymphadenitis [12]. Investigations on the chemical constituents of this plant have yielded saponins, flavonoids, and terpenoids [13], [14], [15], [16]. Dawane et al., 2012 [17] studied the effect of two formulations of E. phaseoloides seeds after topical application in ‘monoiodoacetate-induced osteoarthritis in rats, since arthritis is a very common clinical condition affecting both sexes and all ages. It is rich in protein, minerals [18], which is higher than FAO recommendation.
2. Materials and methods
2.1. Drugs
Imipramine hydrochloride was procured from Sigma–Aldrich Corp., (St Louis, USA). The drug was prepared fresh on the day of the experiment. All other chemicals used were of analytical grade.
2.2. Animals
Healthy male Swiss albino mice weighing between 30.0 ± 5.0 gm were obtained from the animal facility of the Department of Pharmacology & Toxicology, College of Veterinary Science, Khanapara, Assam. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) of College of Veterinary Sciences, Assam Agricultural University, Khanapara (no.770/ac/CPCSEA/FVSc, AAU/IAEC/15-16/367). All the animals were kept in polypropylene cages and had free access to standard balanced ration, clean drinking water ad libitum in standard laboratory conditions (12:12 h light/dark cycle at ambient temperature (22–25 °C)). The animals were acclimatized for two weeks prior to the commencement of the experiment.
2.3. Plant collection and identification
The dried seeds of E. phaseoloides were collected from local market during the month of April–May, 2016 and identified by taxonomist Dr. Iswar Chandra Barua, Principal Scientist, Department of Agronomy, Assam Agricultural University, Jorhat, Assam; a voucher specimen (AAU-NW-EVM-3) was deposited and kept at the herbarium of the Department of Agronomy, Assam Agricultural University, Jorhat-785013, Assam.
2.4. Preparation of methanolic extract
After removing the kernel from the seeds, they were shade dried, powdered mechanically, weighed and stored in airtight container. Then, 250 gm of powdered material was soaked in 1000 ml methanol for 72 h in a beaker and mixture was stirred every 18 h using a sterile glass rod. The Filtrate was obtained three times with the help of Whatman filter paper no.1 and the solvent was removed by a rotary evaporator (BUCHI, R-210, Labortechnik AG, Meierseggstrasse Switzerland) under reduced pressure, leaving a dark brown residue (MEEP). It was stored in airtight container at 4 °C until use. The recovery percentage with respect to dry powder was found to be 26.52% w/w.
2.5. Drug treatments and experimental design
2.5.1. Acute toxicity studies
The acute toxicity studies of methanolic extract of E. phaseoloides were performed according to the Organization of Economic Corporation Development (OECD) Guidelines No.423 by using female albino mice (20–30 g). The extracts were administered orally at 2000 mg/kg to a group of mice (n = 3) and the percentage mortality, if any, was recorded. The animals were kept under observation for the next 14 days for mortality or gross abnormality with the given doses. Based on the acute toxicity study, 100 and 200 mg/kg oral dose were selected for the present study.
2.5.2. Treatment schedule
Animals were divided into five groups (n = 6 per group) and were restrained for 6 h up to 28th days with the help of 50 ml polystyrene tubes. Groups were namely, vehicle control, Restrain, Standard group imipramine (10 mg/kg, i.p.), MEEP (100 and 200 mg/kg, p.o.). Dosing of the extract was started on 22nd day of restrain and continued up to 28th day. The drugs were administered daily 45 min before stress regimen, for 7 consecutive days.
2.6. Behavioural studies
Forced swim test (FST) was performed after 24 h of last restrain to assess the despair behavior of the rodents [19], [20], [21]. Each animal was placed in an inescapable cylinder of diameter 10 cm, filled with water (25 °C) up to 15 cm for the total time period of 6 min, starting 2 min were considered as acclimatization period and observation of last 4 min was taken into account to assess stressful behavior. After every single animal enactment, the water of the cylinder was changed. The activities of animals were videotaped and time of immobility was recorded (Any Maze software, Stoelting Co., USA). The body weight of the animals was taken on the first day of the experiment and on the last day of the experiment. The animals were sacrificed with a high dose of anaesthesia. The brains were immediately removed; hippocampus was dissected out, homogenized with ice-cold phosphate buffer solution (PBS) (pH 8). The homogenates (10% w/v) were centrifuged at 10,000 rpm for 15 min and the supernatant was used for the biochemical estimations.
2.7. Enzyme assays
2.7.1. Assessment of oxidative stress marker and antioxidant status
Lipid peroxidation in the brain homogenate was estimated colorimetrically by thiobarbituric acid reactive substances (TBARS) [22]. The optical density was measured spectrophotometrically at 532 nm (MULTISCAN GO, Themo fisher Scientific). The values were expressed as nM of Malondialdehyde (MDA)/mg of protein. The total protein was estimated by the method of Bradford et al. [23]. Reduced glutathione (GSH) was estimated by Moron et al. [24]. The concentration of GSH was expressed as μg of glutathione/mg protein. SOD was estimated by the method of Marklund and Marklund [25]. The enzyme was expressed in terms of units/mg of protein.
2.8. Quantitative Real Time PCR
The mRNA expression levels of the genes encoding 78 kDa Glucose-regulated protein, 94 kDa Glucose-regulated protein, C/EBP Homologous protein and Caspase-12 in the hippocampus were measured by Real time PCR (7500 Real Time PCR system, Applied Biosystems). The total RNA was isolated from the hippocampus using TRIzol (Ambion) and 1 μg of total RNA was reverse transcribed using Revert Aid First Strand cDNA synthesis kit (Thermo Scientific). Real time PCR was performed using the SYBR Green PCR Master Mix (Thermo Scientific) and amplification was performed using a standard protocol with the Real time PCR system. The Primers adopted from National Centre for Biotechnology Information (NCBI) by Primer BLAST (ILS primers, India) used for amplification were: (Table 1).
Table 1.
List of Oligonucleotide primer sequences for target genes used in Real Time PCR.
| SL No | Gene of interest | Primer sequences | Accession No | Product size (bp) |
|---|---|---|---|---|
| 1. | GRP 78 | Forward 5′- GTTTGCTGAGGAAGACAAAAAGCTC-3′ Reverse 5′- CACTTCCATAGAGTTTGCTGATAATTG-3′ | NC_000068 | 331 |
| 2. | GRP 94 | Forward 5′- TGGGTCAAGCAGAAAGGAG-3′ Reverse 5′- TCTCTGTTGCTTCCCGACTT-3′ |
NM_011631 | 212 |
| 3. | CHOP | Forward 5′- GGAGCTGGAAGCCTGGTATGAGG-3′ Reverse 5′- TCCCTGGTCAGGCGCTCGATTTCC-3′ |
NM_007837 | 150 |
| 4. | Caspase-12 | Forward 5′- GAAGGAATCTGTGGGGTGAA-3′ Reverse 5′- TCAGCAGTGGCTATCCCTTT-3′ |
NM_009808 | 194 |
| 5. | β-actin | Forward 5′- AGCCATGTACGTAGCCATC-3′ Reverse 5′- CTCTCAGCTGTGGTGGTGA-3′ |
NM_007393 | 228 |
The thermal cycler conditions were as follows: 2 min at 50 °C and then 10 min at 95 °C followed by two-step PCR for 40 cycles consisting of 95 °C for 15s and then 60 °C for 1min. All the reactions were performed in duplicate. The results were expressed relative to β-actin as internal control.
2.9. Statistical analysis
Results are expressed as mean ± SEM. Statistical analysis was performed by One way analysis of variance (ANOVA) followed by post hoc Tukey's multiple range tests, using Graph Pad Prism software version 5.0 (San Diego, CA, USA). Results were considered statistically significant when p < 0.05.
2.10. Isolation of compounds from Entadaphaseoloides
The methanolic extract (10 gm) was subjected to column chromatography (silica gel, 100–200 mesh, eluting with hexane/EtOAc mixture of increasing polarity) to give 40 column fractions. Column fractions were analyzed by TLC (silica gel 60 F254, hexane: EtOAc, 60:40), and fractions with similar TLC patterns were combined to give five major fractions (F1, F2, F3, F4, F5). Fractions F4 was subjected to repeated column chromatography eluting with EtOAc: hexane (19:81) to yield compound 1. Fraction F3 was subjected to Column chromatography (CC) on silica gel (100–200 mesh) using a hexane-EtOAc (10:0-6:4) to yield sub fractions B1 and Compound 2. Subfraction B1 was then purified by preparative TLC with CHCl3: MeOH (90:10) to get compound 3. Repeated purification of fraction F4 on silica gel (230–400 mesh) using CHCl3: MeOH (yu90:10) followed by Preparative HPLC, yielded compound 4.
Oleic acid (1): Light yellow oil, 1H-NMR (500 MHz, CDCl3) δ: 5.35 (2H, m), 2.33(4H, m), 2.01 (4H, m), 1.64 (4H, m),1.37–1.22(H, m), 0.88 (3H, t, J = 7.1 & 6.2 Hz). 13C NMR (CDCl3, 75 MHz) δ:180.55, 129.93, 129.63, 34.11, 31.91, 29.76, 29.66, 29.60, 29.53, 29.37, 29.32, 29.14, 29.05, 27.20, 27.13, 24.64, 22.67. HR-ESI-MS m/z: 305.2456 (Calcd for C18H24O2Na: 305.2451).
Entadamide A (2): White amorphous powder, 1H-NMR (500 MHz, CDCl3) δ: 7.62 (1H, d, J = 14.5 Hz), 6.44(1H, br s), 5.69(1H, d, J = 14.6 Hz), 3.71(2H,m), 3.46 (2H, m), 2.32 (3H,s). 13C NMR (CDCl3, 75 MHz) δ:165.80, 143.67, 115.31, 62.46,42.49, 14.60. HR-ESI-MS m/z: 162.0589 (Calcd for C6H12NO2S: 162.0583).
Entadamide A-beta-d-glucopyranoside (3): White amorphous powder, 1H-NMR (500 MHz, CD3OD) δ: 2.33 (3H, s), 3.27 (1H, m), 3.29 (1H, m,), 3.35 (1H, m), 3.37 (1H, m), 3.46 (1H, m), 3.72 (2H, m), 3.90(1H, m), 3.96 (1H, m), 3.99 (1H, m,), 4.29 (1H, d, J = 7.9 Hz), 5.85 (1H, d, J = 14.58 Hz), 7.59 (1H, d, J = 14.58 Hz). 13C NMR (75 MHz, CD3OD): δ 165.58, 142.31, 114.83, 102.39, 75.8, 75.67, 72.97, 69.36, 68.23, 60.74, 38.92, 13.4. HR-ESI-MS m/z: 346.0946 (Calcd for C12H21NO7S: 346.0931).
1-O-protocatechuoyl-β-d-glucose (4): Colour less gummy, 1H-NMR (500 MHz, DMSO-d6) δ:6.91(1H, d, J = 8.68 Hz), 6.59(1H, brs), 6.48 (1H, dd, J = 8.6 & 2.5), 4.47(1H, d, J = 6.8), 3.69(1H, m), 3.47(1H, m), 3.37(1H, m), 3.18(3H, m). 13C NMR (75 MHz, CD3OD) δ:174.56, 152.15, 148.65, 129.14, 117.64, 117.12, 112.84, 103.74, 76.96, 76.45, 73.43, 69.74, 60.88.
3. Results
3.1. Chemistry
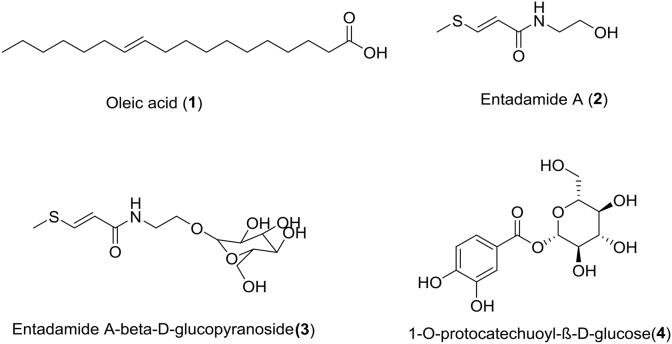
The fractionation and purification led to the isolation of 4 compounds in the crude methanolic extract from the seeds of E. phaseoloides. The structures of isolates were established using IR, MS, 1D and 2D NMR spectroscopic techniques. After comparing their spectral data with those reported in the literature [26] they were identified as known compounds (Fig. 1) and confirmed as Oleic acid (1), Entadamide A (2), Entadamide A-β-d-glucopyranoside (3) and 1-O-protocatechuoyl-β-d-glucose (4).
Fig. 1.
The structures of the most active compounds identified by NMR as followed: Oleic acid (1), Entadamide A (2), Entadamide A-β-d-glucopyranoside (3), 1-O-protocatechuoyl-β-d-glucose (4).
3.2. Effect on immobility time in the forced swim test
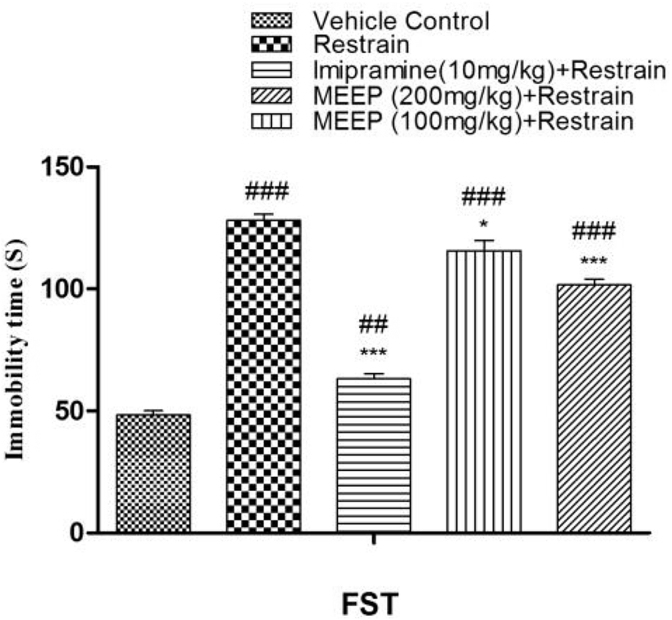
Chronic restrain group showed a behavioral despair revealed by significant (128 ± 2.66 s, p < 0.001) increase in immobility time in FST as compared to vehicle control group (48.30 ± 1.708 s). Pre-treatment with MEEP 100 and 200 mg/kg reduced stress-induced increase in immobility duration (115.7 ± 4.046 s, p < 0.05 and 101.7 ± 2.414 s, p < 0.001). The result is presented in Fig. 2.
Fig. 2.
Effect of methanolic extract of Entada phaseoloides (MEEP 100 and 200 mg/kg) and imipramine pre-treatment on force swimming test in mice in ER stress model. Values represent the Mean ± SEM. of six animals for each group. ###p < 0.001, ##p < 0.01, #p < 0.05 compared with vehicle control. *p < 0.05; **p < 0.01; ***p < 0.001 compared with chronic restrain group.
3.3. Effect of change in body weight
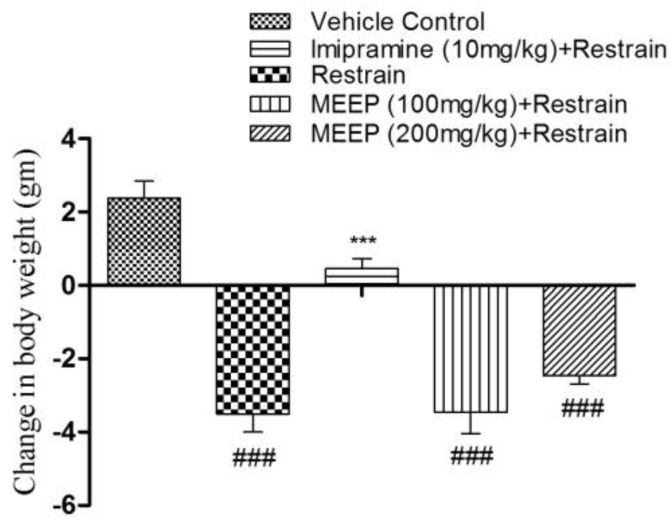
A significant loss in body weight (−3.503 ± 0.495 gm, p < 0.001) was observed in the restrain group when compared with the vehicle control group (2.380 ± 0.465 gm). The standard drug treated group, Imipramine significantly (0.457 ± 0.264 gm, p < 0.001) increased body weight when compared to the chronic restrain group, however, MEEP failed to show positive effect on weight gain at both the doses (MEEP 100 and 200 mg/kg (−3.453 ± 0.583 gm; −2.463 ± 0.22 gm)), signifying weaker activity. The result was presented in Fig. 3.
Fig. 3.
Effect of methanolic extract of Entada phaseoloides (MEEP 100 and 200 mg/kg) and of imipramine (10 mg/kg i.p.) pre-treatment on body weight of mice in ER stress. Values represent the Mean ± SEM. of six animals for each group. ###p < 0.001, ##p < 0.01, #p < 0.05 compared with vehicle control. *p < 0.05; **p < 0.01; ***p < 0.001 compared with chronic restrain group.
3.4. Oxidative stress markers
3.4.1. Effect of MEEP on lipid peroxidation
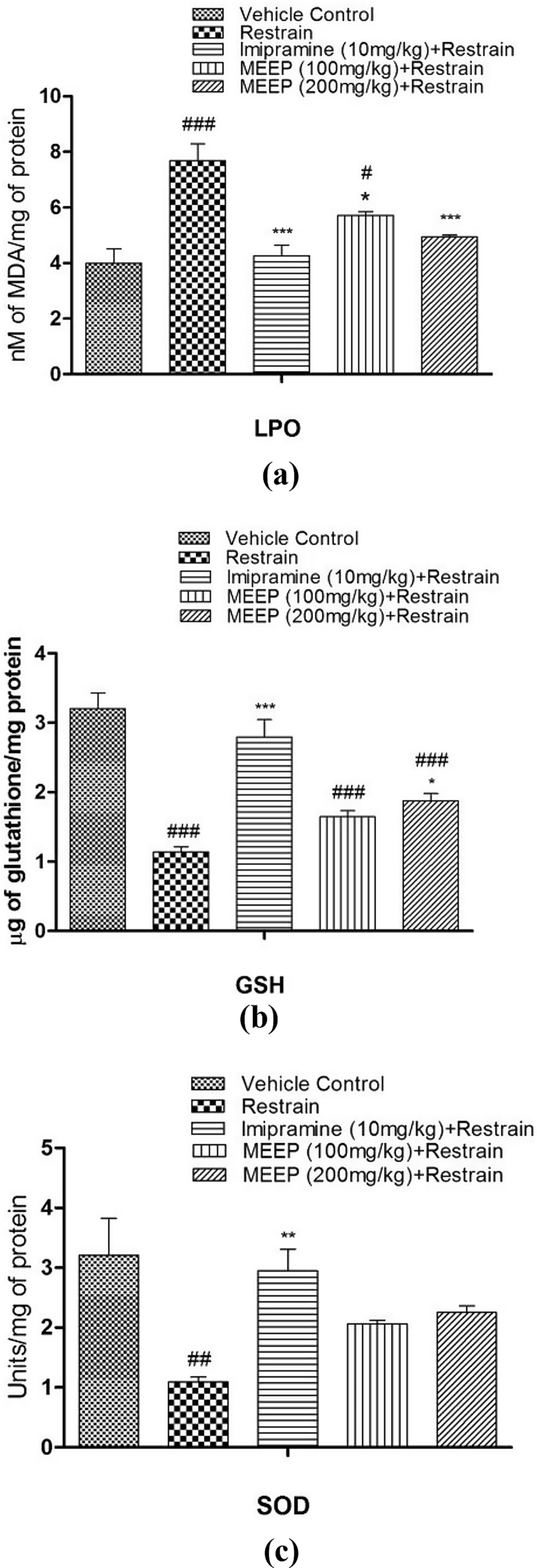
There was significant increase in MDA level (7.68 ± 0.61 nM/mg protein, p < 0.01) in the hippocampus of chronic restrain group when compared to vehicle control group (3.99 ± 0.52 nM/mg protein). Imipramine showed significant (4.25 ± 0.38 nM/mg protein, p < 0.01) decrease in MDA level. MEEP 100 and 200 mg/kg significantly reduced the MDA level (5.712 ± 0.135 nM/mg protein, p < 0.05; 4.95 ± 0.05 nM/mg protein, p < 0.001) when compared to the chronic restrain group. The result is presented in the Fig. 4a.
Fig. 4.
(a–c). Effect of MEEP (100 and 200 mg/kg, p.o.) and Imipramine (10 mg/kg, i.p.) pre-treatment on LPO, GSH and SOD in mice of chronic restrain stress group. Values represent the Mean ± SEM. of six animals for each group. ###p < 0.001, ##p < 0.01, #p < 0.05 compared with vehicle control. *p < 0.05; **p < 0.01; ***p < 0.001 compared with chronic restrain group.
3.5. Antioxidant enzymes
3.5.1. Effect of MEEP on reduced glutathione
There was significant depletion (1.14 ± 0.1 μg/mg protein, p < 0.001) in the level of reduced glutathione in the hippocampus of chronic restrain stress group when compared to vehicle control group (3.20 ± 0.22 μg/mg protein). Imipramine (2.80 ± 0.25 μg/mg protein, p < 0.001) and MEEP 100 and 200 mg/kg, significantly increased (1.646 ± 0.08 μg/mg protein and 1.878 ± 0.1 μg/mg protein, p < 0.05) GSH level when compared to the chronic restrain stress group. The result is presented in the Fig. 4b.
3.5.2. Effect of MEEP on super oxide dismutase
The level of super oxide dismutase was decreased significantly (1.09 ± 0.08 U/mg protein, p < 0.01) in the hippocampus of stress group when compared to vehicle control (3.210 ± 0.61 U/mg protein). Imipramine (2.95 ± 0.36 U/mg protein, p < 0.01) as well as MEEP (100 and 200 mg/kg) showed elevation (2.06 ± 0.06 U/mg protein and 2.25 ± 0.11 U/mg protein) of SOD level than the stress group. The result is presented in the Fig. 4c.
3.6. Quantitative Real Time PCR
3.6.1. Effects of MEEP on GRP 78, GRP 94, CHOP, Caspase-12 gene expression by Real Time PCR
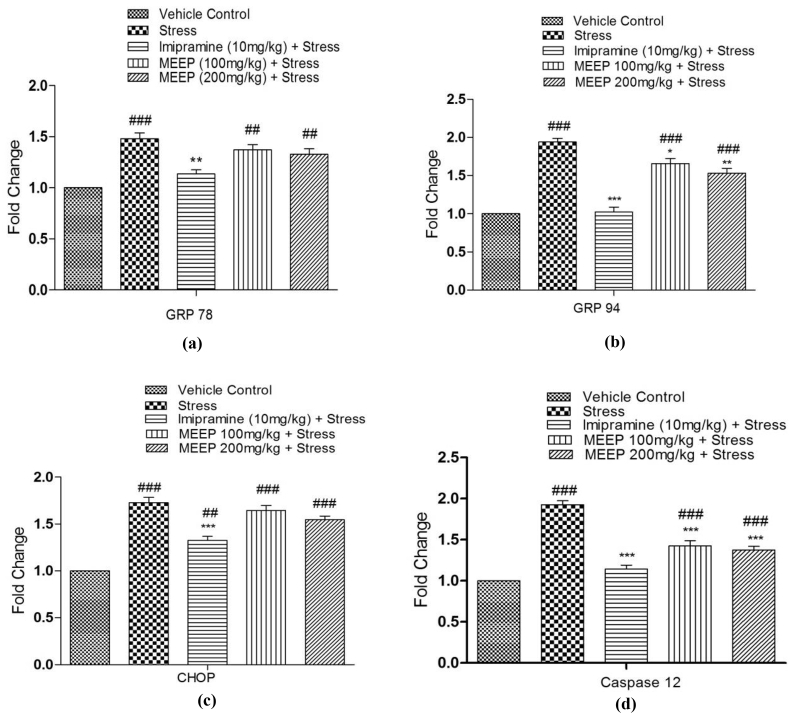
The Quantitative Real Time PCR analysis of GRP 78, GRP 94, CHOP, Caspase-12 gene expression is presented in the Fig. 5(a–d). The Restrain group significantly upregulated the hippocampal gene expressions of the GRP 78 (p < 0.01), GRP 94 (p < 0.001), CHOP (p < 0.001), and Caspase-12 mRNA (p < 0.001) compared with the vehicle control group. However, pre-treatment with MEEP down regulates the mRNA expression of GRP 94 (MEEP 100 mg/kg p < 0.05 and 200 mg/kg; 1.657 ± 0.06 to 1.533 ± 0.06 fold change p < 0.01) and Caspase-12 (MEEP 100 mg/kg; p < 0.001 and 200 mg/kg; 1.423 ± 0.04 to 1.373 ± 0.04 fold change p < 0.001) genes in the hippocampal tissues of mice, similar to that of standard drug Imipramine as compared to the Restrain mice. It also down regulates GRP 78 (1.370 ± 0.05 to 1.327 ± 0.05 fold change) and CHOP (1.643 ± 0.05 to 1.54 ± 0.03 fold change) mRNA expression more significantly in Imipramine treated group rather than MEEP treated groups.
Fig. 5.
(a–d). Quantitative expression of a) GRP 78, b) GRP 94, c) CHOP and d) Caspase-12 genes by Real time PCR in different treatment groups viz. vehicle control, restrain, imipramine, MEEP (100 mg/kg) and MEEP (200 mg/kg) in mice hippocampus with restrain stress. Gene expression was normalized to that of β actin. Values are expressed as fold change represented as Mean ± SEM (n = 3). Statistical significance was determined by one-way ANOVA followed by Tukey's post hoc test ###p < 0.001 when compared to vehicle control; ***p < 0.001 when compared to restrain group.
4. Discussion
Several clinical reports suggest that prolonged exposure to stressful states, which is a common risk factor, could provoke the development of major depression [27], [28], [29]. The progressive hypothalamic pituitary adrenal (HPA) abnormalities caused by traumatic stress or chronic stress trigger behaviors and emotions related to depression and anxiety, which might result in prolonged cortisol hypersecretion resulting neuronal death and hippocampal atrophy observed in depressed individuals [30], [31].
Several studies have demonstrated that different kinds of stress, including restraint stress, cause impairment in the antioxidant status in the brain [32], [33], [34], [35] which could be prevented by antidepressant drugs available commercially; but they generate severe side effects and thus it becomes necessary to develop new drugs with minimum adverse effects and better effectiveness.
In our phytochemical study, 4 compounds have been isolated and identified in the active compound. Oleic acid isolated from the seeds of E. phaseoloides has many positive effects on health. Few reported its usefulness for proper brain function [36], [37]. A study reported 6.2% decline in oleic acid in the postmortem brains of patients who had been suffering from major depressive disorder when compared to normal brain [36]. Another study reported that a diet rich in oleic acid reduces age-related changes in the brain's mitochondria. This might prove that the cytoplasmic concentration of oleic acid will not have any other possible side effects [37]. Consumption of oleic acid replaces other omega fatty acids in the cell membrane as it is less susceptible to oxidation damage. Oleic acid protects the cell membrane from free radical and other oxidative stressors [38]. E. phaseoloides could exert its effect due to its oleic acid component.
Chronic restrain stress-induced depression model is a reliable model for studying depression and has been widely utilized in probing the pathological mechanism of depression and screening of anti-depressant drugs. The present study was carried out to investigate the role of ER stress in depression and to study the effect of imipramine and MEEP (100 and 200 mg/kg, p.o.) on chronic restrain induced depression. The forced swim test is a behavioral despair test which has been used extensively to evaluate depression-like behavior in rodents after exposure to stress. Our investigation showed that mice subjected to chronic stress exhibited significant prolongation of immobility time in this behavioral model. MEEP (100 and 200 mg/kg, p.o.) administration significantly decreased the duration of immobility time in mice in FST indicating anti-depressive like effect.
Body weight of the chronic restrain group also decreased than the vehicle control group; this could be due to an aversion to food and water intake. The standard drug treated group, (Imipramine) significantly increased body weight, but our compound failed to show positive effect on weight gain. Chronic restrain stress stimulates ROS (Reactive oxygen species) which results in the generation of free radicals. Anti-oxidant enzymes are the powerful scavengers of free radicals and act as an inhibitor of oxidative damage. Several studies have demonstrated that the restrained stress significantly elevated lipid peroxidation level in the hippocampus of mice [39]. In context to this, our results showed that the restrain stress procedure caused a significant elevation of lipid peroxidation, as evidenced by the increased amount of TBARS levels in restrain stress mice, which was averted by MEEP and imipramine treatment. Thus, the beneficial effects of MEEP on depressive behavior could be associated with its capacity to prevent the lipid per oxidative damage caused by immobilization stress.
GSH acts as both a nucleophile scavenger of toxic compounds and as a substrate in the Glutathione Peroxidase (GPx)-mediated destruction of hydroperoxides [40]. The depletion of GSH would be expected to compromise this pathway and may thereby allow H2O2 to accumulate to toxic levels. The increase in excitotoxicity-induced free radical load observed under MEEP treatment contributed to a decline in GPx activity and an increase in GSH content in the hippocampus. This decline in GPx activity and elevated GSH content might represent an index of cellular damage prior to the neuronal death. Altogether, these results indicate a potential relationship among several oxidative stress-related parameters in the hippocampus of animals subjected to restrain stress. Restrain stress reduced the SOD activity in the brain; SOD is the only enzyme that uses the superoxide anions as the substrate and produces hydrogen peroxide as a metabolite. Superoxide anion is more toxic than hydrogen peroxide and has to be removed. Pre-treatment with MEEP significantly prevented the reduction of SOD activity in the brain.
GRP 78, CHOP, GRP 94 and Caspase-12 are the classical markers for stress. The C/EBP homologous protein (CHOP, also known as GADD153) is a transcription factor that is activated at multiple levels during ER. CHOP protein is post-translationally activated by the p38 kinase [41]. Deregulated CHOP activity compromises cell viability [42] and cells lacking CHOP are significantly protected from the lethal consequences of ER stress [43], [44]. GRP 78 is an ER chaperone protein regarded as a classical marker of Unfolded Protein Response (UPR) activation. Overexpression of GRP 78 inhibited the upregulation of CHOP, which plays a key role in regulating cell growth and implicated in apoptosis.
Caspase-12 exhibits resistance to ER Stress which plays a role in the process of cell death. Nakagawa et al., (2000) [9] demonstrated that caspase-12 mediated apoptosis was a specific apoptotic pathway of ER and m-calpain, might be responsible for cleaving procaspase-12 [45]. GRP 94, a ubiquitously expressed chaperone, its increased expression indicates ER-stressed cell priming for inflammatory interactions, which is quite relevant in our study in restrain group. Our study depicted that the MEEP can suppress expression of GRP 94 and Caspase-12 genes by recuperating Endoplasmic reticulum stress in chronic restrain stress model in mice. In another study by Jangra et al. (2016), it was reported that Honokiol treatment reverts endoplasmic reticulum stress in mice by down regulating GRP78 and CHOP [21]. Hence mechanism of reverting endoplasmic reticulum stress by MEEP could be different from Honokiol.
We have observed weaker antidepressant-like property in lipopolysaccharide induced depressive behavior in mice. However, memory enhancing activity of the extract showed promising effect. E. phaseoloides, having many Ayurvedic, folk lore and traditional medicinal properties, reverted endoplasmic reticulum stress possibly due to its antioxidant property and down regulating GRP 94 and Caspase-12 genes.
Source(s) of funding
Research works reported in this publication was supported by the grant of the Indian Council of Agricultural Research (ICAR), New Delhi, through “Outreach Project on Ethnoveterinary Medicine” grant no FPA 1-2/2009 JD (Res) dated 16.11.2009.
Conflict of interest
None
Acknowledgement
The authors also express their sincere thanks to the Director of Research (Vety), AAU, Khanapara for providing facility to carry out the work.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Bartolomucci A., Leopardi R. Stress and depression: preclinical Research and clinical implications. PLoS One. 2009;4(1):e4265. doi: 10.1371/journal.pone.0004265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler R.C., Berglund P., Demler O., Jin R., Koretz D., Merikangas K.R. The epidemiology of major depressive disorder: results from the national comorbidity survey replication (NCS-R) J Am Med Assoc. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 4.Kitamura M. Biphasic, bidirectional regulation of NF-kappaβ by endoplasmic reticulum stress. Antioxid Redox Signal. 2009;11:2353–2364. doi: 10.1089/ars.2008.2391. [DOI] [PubMed] [Google Scholar]
- 5.Xu C., Bailly M., Reed J.C. Endoplasmic reticulum stress: cell life and death decision. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang K.K.R. Identification and characterization of endoplasmic reticulum stress-induced apoptosis in vivo. Methods Enzymeol. 2008;442:395–419. doi: 10.1016/S0076-6879(08)01420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherman M.Y., Goldberg A.L. Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron. 2001;29:15–32. doi: 10.1016/s0896-6273(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa T., Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B.A. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 10.Healthy Living Nature LOC localized species and remedies from nature. 2017. [Google Scholar]
- 11.Quattrocchi U, Entandrophragma C, Meliaceae DC. CRC World dictionary of medicinal and poisonous plants: Common names, Scientific Names, Eponyms, Synonyms, and Etymology. 1573.
- 12.Mohan V.R., Janardhanan K. Chemical and nutritional evaluation of raw seeds of the tribal pulses Parkia roxburghii G. Don. and Entada phaseoloides (L.) Merr. Int J Food Sci Nutr. 1993;44:47–53. [Google Scholar]
- 13.Ramakrishna D., Pavan K.K., Mukkanti K., Abedulla K.K. Antiulcer activity of the seeds of Entada phaseoloides. Pharmacology online. 2008;3:93–99. [Google Scholar]
- 14.Ikram M., Babar Z.M., Islam A.M.T., Chowdhury M.A.U., Uddin M.E., Islam M.R. Antidiabetic and hypolipidemic effects of the different fractions of methanolic extracts of Entada phaseoloides (L.) Merr. In alloxan induced diabetic mice. Int J Pharmaceut Sci Res. 2011;2:3160–3165. [Google Scholar]
- 15.Chih Liu W., Kugelman M., Wilson R.A., Rao K.V. A crystalline saponin with anti-tumor activity from Entada phaseoloides. Phytochem. 1972;11:171–173. [Google Scholar]
- 16.Liu R.H., Runyon R.S., Wang Y.C., Oliver S.G., Fan T.P., Zhang W.D. Deciphering ancient combinatorial formulas: the Shexiang Baoxin pill. Science. 2015;347:540–542. [Google Scholar]
- 17.Dawane J., Pandit V., Rajopadhye B., Karandikar M. The effect of two formulations of Entada phaseoloides seeds after topical application in monoiodoacetate-induced osteoarthritis in rats. J Exp Integr Med. 2012;3:37–41. [Google Scholar]
- 18.Barua C.C., Hazorika M., Misra J., Sen S., Bora M., Saikia B.B. Phytochemicals, in vitro antioxidant activity and proximate Composition of seeds of Entada phaseoloides linn. Merrill. Int J Pharm Bio Sci. 2015;6(3):366–376. [Google Scholar]
- 19.Porsolt R.D., Bertin A., Jalfre M. Behavioural despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 20.Can A., Dao D.T., Arad M., Terrillion C.E., Piantadosi S.C., Gould T.D. The mouse forced swim test. J Vis Exp JOVE. 2012;59:3638. doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jangra A., Dwivedi S., Sriram C.S., Gurjar S.S., Kwatra M., Sulakhiya K. Honokiol abrogates chronic restraint stress-induced cognitive impairment and depressive-like behavior by blocking endoplasmic reticulum stress in the hippocampus of mice. Eur J Pharmacol. 2016;770:25–32. doi: 10.1016/j.ejphar.2015.11.047. [DOI] [PubMed] [Google Scholar]
- 22.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 23.Bradford M.M. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 24.Moron S.M., Joseph W.D., Mannervik B. Levels of glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochem Biophys Acta. 1979;582:67–68. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 25.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 26.Yuqiong D., Shi Haiming, Haisha Y., Yunhua P., Mengyue W., Xiaobo L. Antioxidant phenolic compounds from the stems of Entada phaseoloides. Chem Biodivers. 2012;9:68–79. doi: 10.1002/cbdv.201100002. [DOI] [PubMed] [Google Scholar]
- 27.Lloyd C. Life events and depressive disorder reviewed. II. Events as precipitating factors. Arch Gen Psychiatry. 1980;37:541–548. doi: 10.1001/archpsyc.1980.01780180055005. [DOI] [PubMed] [Google Scholar]
- 28.Hill M.N., Hellemans K.G.C., Verma P., Gorzalka B.B., Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev. 2012;36:2085–2117. doi: 10.1016/j.neubiorev.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claes S. Glucocorticoid receptor polymorphisms in major depression. Ann N Y Acad Sci. 2009;1179:216–228. doi: 10.1111/j.1749-6632.2009.05012.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee A.L., Ogle W.O., Sapolsky R.M. Stress and depression: possible links to neuron death in the hippocampus. Bipolar Disord. 2002;4:117–128. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- 31.McKinnon M.C., Yucel K., Nazarov A., MacQueen G.M. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychia Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 32.de Balk R.S., Bridi J.C., de Portella R.L., Carvalho N.R., Dobrachinski F., da Silva M.H. Clomipramine treatment and repeated restraint stress alter parameters of oxidative stress in brain regions of male rats. Neurochem Res. 2010;35:1761–1770. doi: 10.1007/s11064-010-0240-1. [DOI] [PubMed] [Google Scholar]
- 33.Enache M., Van Waes V., Vinner E., Lhermitte M., Maccari S., Darnaudéry M. Impact of an acute exposure to ethanol on the oxidative stress status in the hippocampus of pre-natal restraint stress adolescent male rats. Brain Res. 2008;1191:55–62. doi: 10.1016/j.brainres.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 34.García-Fernández M., Castilla-Ortega E., Pedraza C., Blanco E., Hurtado-Guerrero I., Barbancho M.A. Chronic immobilization in the malpar1knockout mice increases oxidative stress in the hippocampus. Int J Neurosci. 2012;122:583–589. doi: 10.3109/00207454.2012.693998. [DOI] [PubMed] [Google Scholar]
- 35.Kumar A., Goyal R. Quercetin protects against acute immobilization stress-induced behaviors and biochemical alterations in mice. J Med Food. 2008;11:469–473. doi: 10.1089/jmf.2006.0207. [DOI] [PubMed] [Google Scholar]
- 36.Hamazaki K., Hamazaki T., Inadera H. Fatty acid composition in the postmortem amygdala of patients with schizophrenia, bipolar disorder, and major depressive disorder. J Psychiatr Res. 2012;46:1024–1028. doi: 10.1016/j.jpsychires.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 37.Ochoa J.J., Pamplona R., Ramirez-Tortosa M.C., Granados-Principal S., Perez-Lopez P., Naudi A. Age-related changes in brain mitochondrial DNA deletion and oxidative stress are differentially modulated by dietary fat type and coenzyme Q1. Free Radic Biol Med. 2011;50:1053–1064. doi: 10.1016/j.freeradbiomed.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Haug A., Hostmark A.T., Harstad O.M. Bovine milk in human nutrition – a review. Lipids Health Dis. 2007;6:25. doi: 10.1186/1476-511X-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Budni J., Zomkowski A.D., Engel D., Santos D.B., dos Santos A.A., Moretti M. Folic acid prevents depressive-like behavior and hippocampal antioxidant imbalance induced by restraint stress in mice. Exp Neurol. 2013;240:112–121. doi: 10.1016/j.expneurol.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 40.Meister A., Anderson M.E. Glutathione. Ann Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 41.Wang X.Z., Ron D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP-kinase. Science. 1996;272:1347–1349. doi: 10.1126/science.272.5266.1347. [DOI] [PubMed] [Google Scholar]
- 42.Zhan Q., Lord K.A., Alamo, Hollander M.C., Carrier F., Ron D. The Gadd and MyoD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol Cell Biol. 1994;14:2361–2371. doi: 10.1128/mcb.14.4.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zinszner H., Kuroda M., Wang X., Batchvarova N., Lightfoot R.T., Remotti H. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oyadomari S., Araki E., Mori M. Endoplasmic reticulum stress-mediated apoptosis in pancreatic β-cells. Apoptosis. 2002;7:335–345. doi: 10.1023/a:1016175429877. [DOI] [PubMed] [Google Scholar]
- 45.Rao R.V., Hermel E., Castro-Obregon S., Ellerby L.M., Ellerby H.M., Bredesen D.E. Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J Biol Chem. 2001;276:33869–33874. doi: 10.1074/jbc.M102225200. [DOI] [PubMed] [Google Scholar]