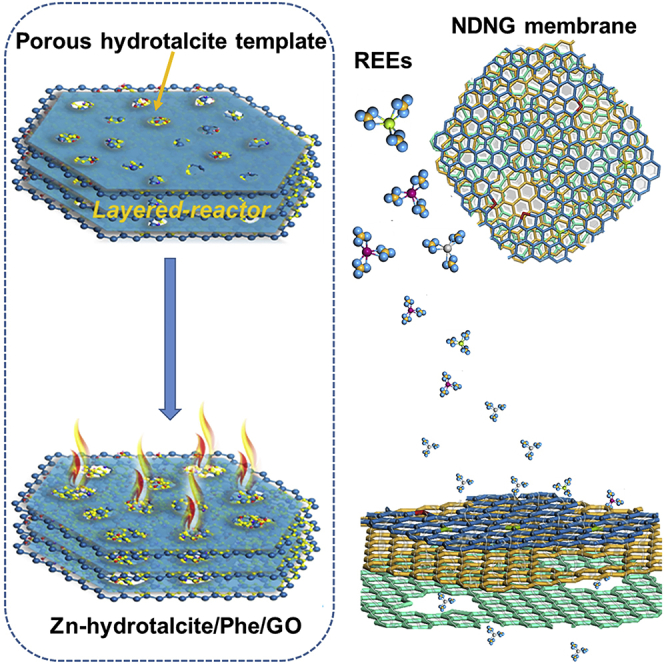
Summary
Rare earth separation is still a major challenge in membrane science. Nitrogen-doped nanoporous graphene (NDNG) is a promising material for membrane separation, but it has not yet been tested for rare earth separation, and it is limited by multi-complex synthesis. Herein, we developed a one-step, facile, and scalable approach to synthesize NDNG with tunable pore size and controlled nitrogen content using confinement combustion. Nanoporous hydrotalcite from Zn(NO3)2 is formed between layers of graphene oxide (GO) absorbed with phenylalanine via confinement growth, thus preparing the sandwich hydrotalcite/phenylalanine/GO composites. Subsequently, area-confinement combustion of hydrotalcite nanopores is used to etch graphene nanopores, and the hydrotalcite interlayer as a closed flat nanoreactor induces two-dimensional space confinement doping of planar nitrogen into graphene. The membrane prepared by NDNG achieves separation of Sc3+ from the other rare earth ions with excellent selectivity (∼3.7) through selective electrostatic interactions of pyrrolic-N, and separation selectivity of ∼1.7 for Tm3+/Sm3+.
Subject areas: Separation Science, Chemical Synthesis, Porous Material
Graphical Abstract
Highlights
-
•
A multiple confinement strategy is constructed to achieve the synthesis of NDNG
-
•
Planar nitrogen-doped NDNG with tunable pore size is obtained by one-step synthesis
-
•
NDNG membrane presents excellent selectivity for rare earth in strong acidic media
Separation Science; Chemical Synthesis; Porous Material
Introduction
Rare earth elements (REEs) are critical for designing optical, electrical, and magnetic functional materials because of their unique properties, which are therefore referred to as "industrial vitamin", and listed as strategic materials (Hu et al., 2018). REEs are very similar in their physicochemical properties, and exist in the form of associated ore deposits, suggesting that the separation and purification of individual REEs are necessary but extremely difficult (Cheisson and Schelter, 2019; Wang et al., 2018). The existing separation techniques of REEs involve cascade extraction (Brigham et al., 2017), chromatographic separation (Florek et al., 2016), and electrolytic separation technologies (Cheisson and Schelter, 2019) with the problems of complex operations, numerous procedures, high energy consumption, and low efficiency. In comparison, the membrane separation technology without phase change or additives has gained considerable attention owing to its simple, low carbon emission, easy operation, and suitable for industrial automation applications (Chen et al., 2017; Seo et al., 2018; Werber et al., 2016). However, it is still a challenge to use membrane technology for effective separation and purification of REEs because of the lack of appropriate materials (Chen et al., 2018).
The membrane of graphene oxide (GO) as a class of emerging membranes has been fabricated by stacking a large number of GO fragments with a controlled interlayer for precise ionic and molecular sieving (Chen et al., 2017; Huang et al., 2014; Joshi et al., 2014; Lipton et al., 2020). However, the low penetration rate of ions resulting from the limited interlayer spacing of GO membrane hinders the development in practical applications (Cohen-Tanugi and Grossman, 2012). As a very promising material, nitrogen-doped nanoporous graphene (NDNG) has attracted much attention (Ai et al., 2015; Bian et al., 2020; Li et al., 2017; Men et al., 2016; Pawlak et al., 2020; Sint et al., 2008; Wu et al., 2020; Yan et al., 2017). The presence of in-plane nanopores shortens the penetration path of ions to significantly enhance ion penetrability, and the incorporation of nitrogen into the graphene lattice enhances the electron-donor tendency of the nanoporous graphene for increasing separation selectivity toward ions. In addition, NDNG as carbon-based materials has high stability in strong acidic environments, thus it is expected to achieve the selective and efficient separation of REEs through NDNG membranes.
The synthesis technologies of NDNG from GO usually involve two independent steps (Bian et al., 2020; Men et al., 2016; Wu et al., 2020). The first step is the physical or chemical pore-making process with high energy-consuming and complex pre-preparation of the template, and the second step is a complex and long chemical doping process in a high-temperature sealed system. Unfortunately, the existing technologies of making pores are difficult to integrate with heteroatom doping for achieving a one-step synthesis of NDNG. Thus, the production of NDNG still suffers from complexity, low efficiency, and time-consuming, and it remains challenging until today.
In this work, we developed a new one-step combustion approach through a multiple confinement strategy to prepare NDNG with high separation selectivity toward REEs. Interlayer space of multilayer GO membrane was used for confined growth of Zn-hydrotalcite to construct sandwich composites of hydrotalcite/phenylalanine/GO. Nitrogen atoms from phenylalanine (Phe) were then doped into the graphene lattice between layers of Zn-hydrotalcite through the two-dimensional (2D) space confinement combustion, and the as-formed porous Zn-hydrotalcite was also used as a template to etch nanopores on GO via pore-area confinement combustion. NDNG with a large amount of planar nitrogen and tunable pore size was prepared as a membrane for REEs separation, and showed excellent selectivity. This discovery not only establishes a new route to prepare the heteroatom-doped nanoporous graphene, but also opens a new avenue for realizing high-performance REEs separation membranes.
Results and discussion
Design and synthesis of NDNG
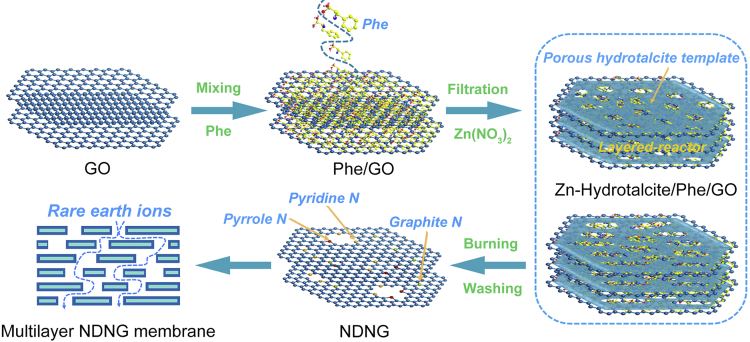
The procedure for the synthesis of NDNG is illustrated in Scheme 1. Phe, as an aromatic and natural amino acid, is low cost and easily absorbed by GO via π–π stacking interaction (Ahn et al., 2014; Wang et al., 2014), thus it was selected to be the nitrogen doping precursor. As described in Scheme 1, after mixing uniformly of GO and Phe, the Phe molecules with benzene ring were basically parallel to the GO sheets forming well-ordered molecular films by filtering with filter paper (Krebs et al., 2018). Simultaneously, Zn(NO3)2 and H2O were intercalated into the layers of as-formed Phe/GO membrane, which caused the 2D-space confinement self-controlled growth of Zn-hydrotalcite, and thus a sandwich composite of Zn-hydrotalcite/Phe/GO was prepared successfully. After burning and washing, NDNG with controllable pore size and nitrogen doping content can be obtained. More experiment details can be found in Supporting Information.
Scheme 1.
Synthesis of NDNG through multiple confinement strategy and then NDNG membrane was prepared for rare earth elements separation
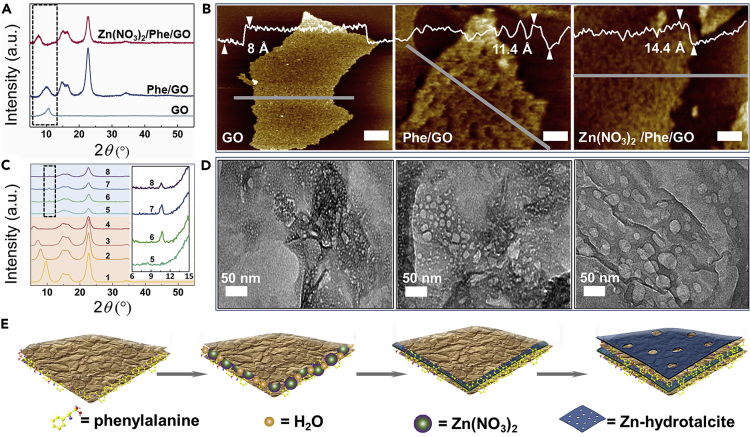
In order to confirm the formation of Zn-hydrotalcite/Phe/GO composites, the reaction process before combustion synthesis was monitored by X-ray diffraction (XRD). As shown in Figure 1A, the peak at ∼10.7° is of (001) reflection of GO sheets, which is corresponding to the interplanar spacing of 8.3 Å, and the peaks at ∼15°, 16.5°, 22.7°, and 34.2° are from the filter paper (Tan et al., 2020b). As compared to GO sheets, the (001) diffraction peak of GO in composites of Phe/GO and Zn(NO3)2/Phe/GO has shifted to smaller angles ∼10.1° and 7.6°, and corresponding interlayer spacing increases to ∼8.8 Å and 11.5 Å, respectively (Figure S1A). Therefore, Phe and Zn(NO3)2 have been successfully inserted into the layers of GO, which causes an increase of the interplanar spacing of GO. The corresponding morphological information was obtained by atomic force microscopy (AFM), as shown in Figure 1B. AFM image of GO shows an unfolding 2D nanosheet, and the thickness of ∼8 Å is consistent with that of monolayer GO (Xu et al., 2010). Moreover, the thickness of the composites Phe/GO and Zn(NO3)2/Phe/GO is 11.4 Å and 14.4 Å, respectively. The increased thickness of ∼3.4 Å and 6.4 Å can result from the adsorption of Phe and Zn(NO3)2/Phe onto both sides of GO, respectively. Moreover, the uneven surface of the composites Phe/GO and Zn(NO3)2/Phe/GO with many nanopores can be observed clearly, which indicates that Phe and Zn(NO3)2 are not uniformly and completely covered on the GO basal plane but forming 2D porous layers. Thus the Zn(NO3)2/Phe/GO composite with sandwich structure was constructed successfully. For studying the forming process and structure of porous layers on the GO sheets, we analyzed XRD patterns of the samples Zn(NO3)2/Phe/GO prepared with different concentrations of Zn(NO3)2 as shown in Figure 1C. With the concentration of Zn(NO3)2 increasing from 50 to 200 g/L, the peak position of the (001) plane is shifted gradually to the low 2θ value from 9.6° to 5.7°, which corresponds to the increase of interlayer distance of GO from 9.2 to 15.4 Å caused by simultaneous intercalation of Phe and Zn(NO3)2 (Figure S1B). Meanwhile, the diffraction peak intensity of the (001) plane gradually decreases and the breadth of the peak increases with the increasing of the amount of Zn(NO3)2 in the hybrids, indicating an increase of the amorphous phase content.
Figure 1.
Fabricating mechanism of Zn-hydrotalcite/Phe/GO composites with sandwich structure
(A) XRD patterns of GO and GO composites with filter paper. The 2θ range for the (001) peak of the GO is highlighted in the dashed box.
(B) AFM images of GO nanosheet and GO composites samples. Scale bars are all 100 nm.
(C) XRD patterns of the composites Zn(NO3)2/Phe/GO at concentrations of Zn(NO3)2 = 50 g/L, 100 g/L, 150 g/L, 200 g/L, 300 g/L, 400 g/L, 450 g/L and 500 g/L (1–8), respectively. Insert: The X axis expansions in the 6–15° 2θ diffraction angle regions in the dashed box (5–8).
(D) TEM images of the composites Zn(NO3)2/Phe/GO at concentrations of Zn(NO3)2 = 200 g/L, 400 g/L, and 500 g/L from left to right, respectively.
(E) Schematic showing the formation of sandwich composites of Zn-hydrotalcite/Phe/GO.
The original GO peak completely disappears at the Zn(NO3)2 concentration of 300 g/L, meaning that the GO layers are fully intercalated with Phe and Zn(NO3)2, and the intercalated structure is no longer orderly arranged (Dimiev and Tour, 2014). Meanwhile, the new signal appears at 10.6° 2θ corresponding to an interlayer distance of 8.4 Å in the sample, which is indicative of the formation of a new crystalline phase. According to the previous report, the characteristic peak of the new phase can be indexed to the (003) plane of hydrotalcite (Garcia-Gallastegui et al., 2012), which we define as Zn-hydrotalcite covering GO. In the previous work, theoretical calculation showed that a cyclic structure can be formed between nitrate ions and water molecules by hydrogen bonding (Li et al., 2018). This distribution pattern leads to the formation of a layered structure of ions and water molecules parallel to the graphene surface. After filtration, the layered structure can be remained between the graphene sheets. Algara-Siller et al. reported that the pressure between graphene layers is up to 1 GPa, which can induce graphene interlaminar water to become 2D ice (Algara-Siller et al., 2015). Thus, it can be inferred that the formation of Zn-hydrotalcite between GO layers can also be attributed to the high press. Most notably, the Zn-hydrotalcite structure formed in the GO interlayer galleries Inherits the ordered stacking of constituent graphene layers. Therefore, the stacking Zn-hydrotalcite/Phe/GO composite with sandwich structure is also confirmed by XRD patterns. With the increase of the amount of Zn(NO3)2 from 300 g/L to 500 g/L, the peak position remains unchanged but its intensity shows a trend from rising to decline, reaching the highest point at 400 g/L. The effect may be attributed to an increase in the integral lateral area of hydrotalcite, and then a mass of emerging pores on the Zn-hydrotalcite surface reduces its crystallinity (Garcia-Gallastegui et al., 2012; Li et al., 2018). These results also can be confirmed by transmission electron microscope (TEM) images as shown in Figures 1D and S2. First, with the increase of the amount of Zn(NO3)2 from 0 to 100 g/L, black covering on GO gradually deepens, and transparency of composite Zn(NO3)2/Phe/GO reduces. Then, some defective pores covering the GO surface can be observed at the amount of Zn(NO3)2 of 150 g/L (Figure S2). As shown in Figure 1D, the pore size on the surface of composites was easily regulated from small to large by increasing the concentration of Zn(NO3)2 from 200 g/L to 500 g/L. The results also show that the lateral size of the Zn-hydrotalcite depends on the salt concentration on the surface of Phe/GO after filtering. Therefore, there are many nanopores with tunable size on the surface of layered Zn-hydrotalcite, which can be used as nanotemplate for making pore through combustion etching. Meanwhile, a closed 2D interlayer nanospace between the layered Zn-hydrotalcites acts as nanoreactors to incorporate nitrogen from Phe into the graphene lattice to prepare NDNG (Figure 1E).
Morphological and structural characterizations
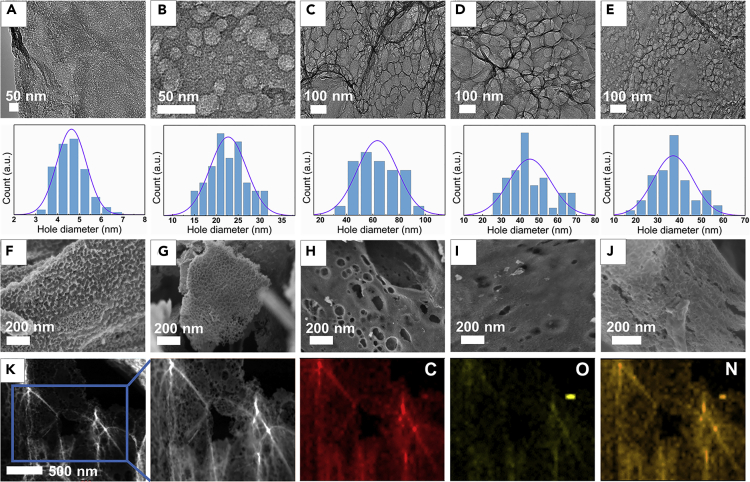
The pore size of the membrane and nitrogen content may be the key factors affecting the permeation and separation of REEs. Therefore, the concentration of Zn(NO3)2 in the reactant was adjusted to be 200, 400, and 500 g/L for controlling the pore size of NDNG to obtain NPNG-1, 2, and 3, respectively. Subsequently, the adding amount of Phe is decreased from 15 g/L to 10 g/L and 5 g/L for obtaining NPNG-3, 4, and 5, respectively. After combustion treatment and washing, NDNG with different pore size and nitrogen content can be obtained as shown in Figure 2. The samples of NDNG-1, 2, and 3 show an average pore size of ∼5, ∼23, and ∼65 nm, respectively (Figures 2A–2C, 2F-H), and the samples of NDNG-3, 4, and 5 exhibit the similar pore size (Figures 2C–2E, 2H-J). Thereby, it can be known that the morphology and size of pores on NDNG are dependent only on the addition amount of Zn(NO3)2. The element distribution of NPNG was performed by TEM with the corresponding EDS mappings. As shown in Figure 2K, N atoms are distributed uniformly and well-doped on the base plane, and around the pores of NDNG. In addition, the distribution intensity of O mappings is much lower than C and N, indicating that O atoms may be replaced with N atoms, or pyrolyzed during combustion. For all the NDNG samples, the XRD pattern undergoes significant changes as compared with pre-combustion composites (Figure S3). Only one broad pattern appears at 2θ = 26°, which is corresponding to the interlayer distance of 3.4 Å originating from the (002) plane of reduced GO (Du et al., 2015; Song et al., 2019). These results also agree with the above element distribution that most oxygen-functional groups were replaced and removed after burning. Raman spectra in Figure S4 is used to provide the additional structural information. Two peaks can be observed at ∼1335 cm−1 and 1596 cm−1, which are corresponding to the D band and G band in the graphene, respectively. The intensity ratio of D band to G band (ID/IG) can be used to characterize the disorder degree (Lin et al., 2012; Tan et al., 2020a). Apparently, a clear increase of ID/IG from NDNG-1 to NDNG-3 can be found from 0.97 to 1.30 with the increase of the pore size, which is consistent with previous reports (Zhou et al., 2014). Moreover, ID/IG ratios decrease slightly from 1.30 to 1.10 for NDNG-3 and NDNG-5, indicating that the incorporation of N atoms into the graphene carbon framework can increase the defective degree of NDNG (Lin et al., 2012). In order to evaluate the accessible surface of NDNG, N2 adsorption-desorption isotherms are recorded in Figure S5. The Brunauer-Emmett-Teller surface area of NDNG-1, 2, and 3 is ∼352, 579, and 586 m2/g, respectively (Figure S5A). Therefore, the specific surface area of NDNG increases with the increase of pore size, which indicates that the resulting pore can improve significantly the accessible surface. As can be seen from Figure S5B, the specific surface area of NDNG-3, 4, and 5 is ∼586, 501, and 500 m2/g, respectively, suggesting that the introduction of nitrogen atoms increases slightly the surface area of NDNG. Fourier transform infrared (FT-IR) spectroscopy and thermogravimetric analysis (TGA) of different samples including GO, Phe/GO, Zn-hydrotalcite/Phe/GO and NDNG-3 also show that NDNG was prepared successfully (Figures S6 and S7).
Figure 2.
TEM and SEM images of NDNG
(A–J) TEM (A–E) and SEM (F–J) images of various NDNG prepared by different concentration of Zn(NO3)2 and Phe. (A, F) NDNG-1 (Zn(NO3)2: 200 g/L; Phe: 15 g/L). (B, G) NDNG-2 (Zn(NO3)2: 400 g/L; Phe: 15 g/L). (C, H) NDNG-3 (Zn(NO3)2: 500 g/L; Phe: 15 g/L). (D, I) NDNG-4 (Zn(NO3)2: 500 g/L; Phe: 10 g/L). (E, J) NDNG-5 (Zn(NO3)2: 500 g/L; Phe: 5 g/L).
(K) TEM image of NDNG-3 combined with EDS mapping in the same area and relative intensities of C (red), O (yellow), and N (orange) elements.
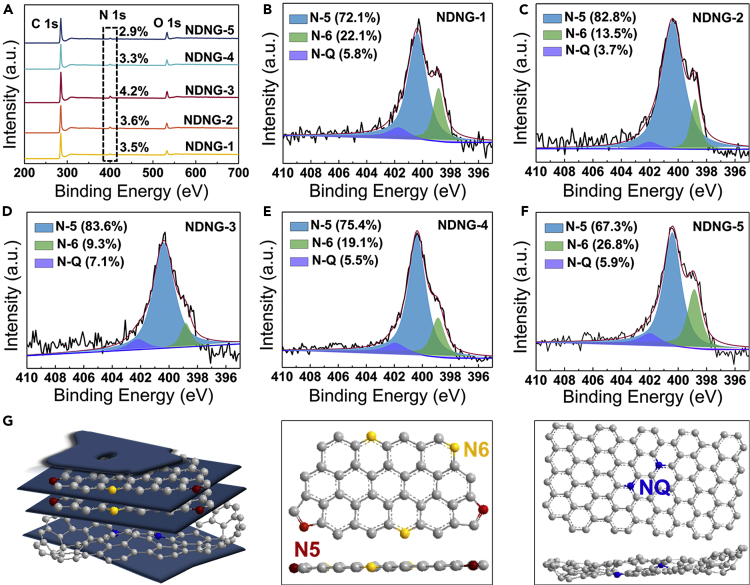
The elemental composition and bonding configurations of NDNG are characterized by X-ray photoelectron spectrometer (XPS) as shown in Figure 3. From XPS spectra (Figure 3A), it can be found that the major component in the as-prepared samples is C (284.6 eV) and followed by heteroatoms including N (397.1–402.9 eV) and O (530–534 eV). The nitrogen doping content significantly decreases from 4.2% for NDNG-3 to 3.3% for NDNG-4, and to 2.9% for NDNG-5, which indicates that the nitrogen content of NDNG can be controlled by adjusting the addition amount of Phe. As shown in Figure S8, the C 1s spectrum is deconvoluted into four peaks at 284.8, 285.5, 286.5, and 288 eV, corresponding to C-C, C=N/C-O, C-N/C=O, and O-C=O, respectively (Lin et al., 2012). The presence of C-N or C=N bond further confirms the formation of the N-doped graphite structure of the prepared samples. The high-resolution spectrum of N 1s for NDNG could be deconvoluted into three peaks at 398.5 eV, 400 eV, and 401.2 eV, which correspond to pyridinic (N6), pyrrolic (N5), and quaternary nitrogen (NQ) (Men et al., 2016), respectively (Figures 3B–3F). N6 and N5 are considered as planar sp2 hybridized nitrogen, and NQ is thought to be tetrahedral sp3 hybridized nitrogen (Figure 3G) (Ding et al., 2013). After combustion treatment, NQ species only accounts for 5.8, 3.7, 7.1, 5.5, and 5.9% of the N species in NDNG-1, NDNG-2, NDNG-3, NDNG-4, and NDNG-5, respectively. It is worth noting that NDNG-2 is doped with a high planar-nitrogen content of 96.3%. These results can be attributed to the space-confinement doping of nitrogen between the Zn-hydrotalcite 2D layers, especially resulting from the largest crystallite size of the Zn-hydrotalcite with Zn(NO3)2 concentration of 400 g/L. Therefore, it can be inferred that nitrogen doping of GO caused by nanospace-confinement from Zn-hydrotalcite can selectively produce planar N6 and N5 in NDNG and constrains the formation of NQ dopant via steric hindrance effect, which is very corresponding with the previously reported mechanism (Ding et al., 2013). For comparison, alanine (Ala) was chosen as another kind of nitrogen doping agent, which is similar to Phe but without the phenyl ring in chemical structure. As shown in Table S1, the combustion products obtained with different adding order of amino acid and salt into GO suspension exhibit different N doping amount in the approximate order: Zn(NO3)2/Phe/GO > Phe/Zn(NO3)2/GO > Zn(NO3)2/Ala/GO > Ala/Zn(NO3)2/GO. The higher N doping amount of NDNG prepared by Phe may result from a higher adsorption amount of Phe adsorbed on the GO surface, meaning that the interaction between GO and aromatic Phe is stronger than that between GO and non-aromatic Ala. Moreover, the N doping amount of NDNG prepared by Zn(NO3)2/amino acid/GO adding order is higher than the one prepared by amino acid/Zn(NO3)2/GO, suggesting that the adding order also could affect the N doping content of NDNG. According to the above confinement combustion mechanism, only amino acid molecules between Zn-hydrotalcite layers can be mostly incorporated into the graphene lattice. Therefore, as compared to the addition of Zn(NO3)2 before amino acids into GO suspension, the addition of amino acids before Zn(NO3)2 can cause more amino acid molecules to be sandwiched between layers of Zn-hydrotalcite after filtering and drying, thereby synthesizing NDNG with higher nitrogen doping amount after burning, and this also means that nitrogen loss during combustion can be alleviated by the formation of Zn-hydrotalcite.
Figure 3.
XPS spectrum of NDNG
(A) Survey spectrum of NDNG.
(B–F) Typical high-resolution XPS N 1s spectra of NDNG.
(G) Schematic representation showing the selectivity for planar N during NDNG synthesis.
Separation of REEs
Currently, membrane strategies for REEs separation are mainly classified into two types: liquid membranes and solid membranes (Chen et al., 2018). During the last few decades, liquid membranes demonstrate efficient permeation and separation performances toward metal ions on account of its large mass transfer interfacial areas (Anitha et al., 2013; Kim et al., 2016; Pei et al., 2011), but it got no breakthrough in scale-up experiments due to poor stability. In this regard, solid membranes are more promising because the solid ones are more stable, and suitable for industrial applications (Chen et al., 2018). The current interests in solid membrane technique for REEs separation are mainly in polymer inclusion membranes and some ion imprinted techniques on polymeric membranes (Croft et al., 2018; Hong et al., 2014; Yusoff et al., 2017; Zheng et al., 2017). However, the major problem with most polymer-based solid membranes is the weak stability and susceptibility in a concentrated acid environment of REEs hydrometallurgy process. Considering the separation nature of membranes, the high porosity, surface area and the abundance of surface nitrogen atoms of the NDNG inspired us to solve a longstanding problem of REEs separation in strong acidic media.
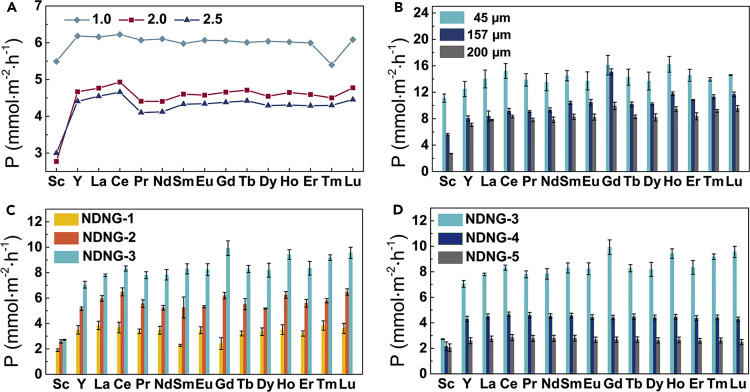
The resulting NDNG-1, NDNG-2, NDNG-3, NDNG-4, and NDNG-5 were prepared as membrane by vacuum filtration for studying its separation selectivity toward REEs. As shown in Figures S9A–S9E, the uniform surfaces of the membranes can be clearly observed. The scanning electron microscope (SEM) characterizations of the cross-section morphology of the membranes are shown in Figures S9F–S9J, which reveal that the as-prepared NDNG membranes possess a lamellar structure. Subsequently, the test membranes were fixed between the feed and driven tanks for performing a series of ion-penetration experiments in Figure S9K. Because industrial processes involving REEs separation are likely to be conducted in acidic conditions (Wang et al., 2018), a pH of 3 was selected in the feed solution for all experiments unless otherwise stated. As shown in Figure S10, the concentration of REEs (Sc3+ and Gd3+) in the driven solution increases significantly in a near-linear manner over time. The NDNG membrane still maintains higher selectivity for REEs (Sc3+ and Gd3+) after separation for 48 hr, which indicates that the separation performance of membrane is stable under the filtration conditions. The Gd3+ permeates efficiently through the NDNG-1 membrane, whereas Sc3+ infiltrates much more slowly. Subsequently, the penetration and selectivity capacity of the NDNG-1 membrane were evaluated in a series of driven solutions with different pH values. According to previous reports, REEs begin to precipitate as hydroxides out of solution at pH > 4 (Giret et al., 2018; Wang et al., 2018). As shown in Figure 4A, in the effective range (pH < 3), a high concentration of H+ in the driven solution could promote significantly the permeation of REEs through NDNG-1 membrane, indicating ion exchange during the separation process (Li et al., 2016). The lowest infiltration concentration of Sc3+ was achieved at a pH of 2.0 of the driven solution, and the highest separation factor can be obtained between Sc3+ and other REEs, meaning that pH can be a controllable parameter to improve the permeation selectivity of NDNG for REEs. In order to further explore the effect of NDNG on the separation of the REEs, we assessed the permeation properties of NDNG membranes with different thicknesses. As shown in Figure 4B, the flux of the REEs always reduces with the increasing membrane thickness, which could be ascribed to a growth in the diffusion hamper or resistance properties of membranes (Fang et al., 2016; Meng et al., 2017). Here, the maximal separation factor increased from 1.5 to 2.9 with the increase of the thickness of NDNG-1 membranes (Figure S11), which can be attributed to the increased interaction sites (Huang et al., 2013). In addition, a huge difference can be found in the permeation ability of different NDNG membranes in the sequence NDNG-3 > NDNG-2 > NDNG-1 (Figure 4C), which can be ascribed to the increase of pore size of NDNG. The selective separation behavior of Sc3+ from other REEs through the NDNG membrane is investigated in Figure S12A. Interestingly, the separation factor of M/Sc increases with the increase of pore size, and the largest separation factor is about 3.7 corresponding to Gd3+/Sc3+. As shown in Table S2, the separation performance of the NDNG membrane is very similar to that of ion imprinted membranes (Cui et al., 2019; Li et al., 2015; Liu et al., 2014, 2017; Lu et al., 2018; Zheng et al., 2017, 2018). Because of the large pores in NDNG-3 (∼65 nm), the selectivity for Sc3+ from other REEs cannot be attributed to the special pore structure and ionic-radius-based theories (Sun et al., 2013). It is therefore inferred that the electrostatic attractions and chemical interactions between functional groups of NDNG sheets and hydrated ions may be responsible for the selectivity of membranes toward REEs. Based on the above XPS analysis, combining high permeability and high selectivity of NDNG-3 membrane to REEs may be attributed to the highest percentage of N5 in N species in NDNG-3 (>83%). As shown in Figures 4D and S12B, both permeation ability and selectivity decrease with a decrease in the N-doping content of NDNG, indicating that the doping of nitrogen atoms has an important impact on the separation of REEs. Further analysis revealed in Figure S13 shows that NDNG-3 could also be used to achieve the selective separation between lanthanide elements. The highest selectivity is calculated as ∼1.7 corresponding to Tm3+/Sm3+. As we know, the long-term stability of membranes is critically important for industrial applications (Thebo et al., 2018). Compared with liquid membranes (Chen et al., 2018), the NDNG membranes are very stable and remain their original structure in acidic (pH = 3.0) solutions even after 90 days (Figure S14). In short, the NDNG membrane with high permeation and separation ability and strong acid-resistant ability has great potential in application.
Figure 4.
Permeation and separation of REEs through NDNG membranes
(A) Effect of pH on the separation for NDNG-1 membrane. The pH of feed solution of REEs (5 mM) was adjusted to 3.0. The pH of driven solution was adjusted by diluted NH4OH or HNO3 solution. The thickness of the membranes is 105 μm.
(B) Permeation flux of REEs as a function of the NDNG-1 membrane thickness. The feed solution has a concentration of 5 mM for all elements at a pH of 3.0; The driven solution is dilute nitric acid at a pH of 2.0.
(C and D) Permeance with different NDNG membranes (NDNG-1, NDNG-2, NDNG-3, NDNG-4, and NDNG-5) for the REEs. The feed solution has a concentration of 5 mM for all elements at a pH of 3.0; The driven solution is dilute nitric acid at a pH of 2.0. The thickness of the membranes is 105 μm.
Theoretical simulation
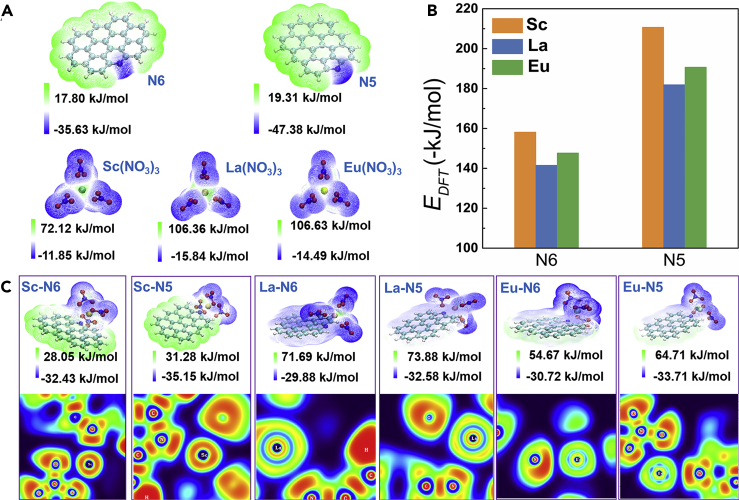
Finally, the density functional theory (DFT) calculations were further performed to illustrate the underlying mechanism taking place during the ion transportation and separation process. According to our XPS results, the N configuration in the as-prepared NDNG is dominated by N6 and N5. Therefore, the electrostatic potential (ESP) on van der Waals surface is calculated of N6 and N5 on the sheets of NDNG and nitrate of Sc, La, Eu, respectively. Figure 5A shows that the site of N5 possesses a more negative ESP than that of N6, which indicates that N5 has a stronger ability to attract REEs by electrostatic force. Subsequently, the DFT electronic energy (EDFT) was calculated to illustrate the interactions between cations and two types of planar nitrogen atoms. It is apparent that for all the complex systems, the N5 presents a stronger binding energy with three metal ions (from ∼-211 to ∼ -191 kJ/mol) than N6 (from ∼-158 to ∼ -148 kJ/mol), which confirms that the N5 plays a leading role in the process of separation in this work. More importantly, it is calculated that the interaction energies of Sc3+ ion both on N6 and N5 are more negative relative to La3+ and Eu3+ (Figure 5B), which can be used to explain the flux of Sc3+ is always lower than that of the other REEs. In addition, to further give a clear and quantitative description of the feasible chemical bond between ions and N atoms, electron location function analysis was used in this work. Figure 5C shows that the interactions between the ions and N atoms are mainly electrostatic interactions without the formation of chemical bonds. Therefore, the strength of electrostatic interactions plays a decisive role in defining the ultimate separation performances. As far as the transport properties are concerned, solid membranes commonly present two kinds of transport mechanisms: facilitated and retarded transport (Chen et al., 2018). In order to exclude the adsorption effect, the maximum adsorption capacity for NDNG-3 was calculated from adsorption isotherm experiments at pH 3. For Sc3+, the equilibrium isotherm was established and fitted well with Langmuir model as shown in Figure S15. The parameters of the corresponding linear regressions are summarized in Table S3. The Freundlich model was also used but did not fit to the experimental isotherm data. The experimental maximum adsorption capacity of NDNG-3 for Sc3+ is calculated to be 1.44 mg/g in the presence of all other REEs at the same concentration. The concentration corresponding to the maximum adsorption capacity is considerably lower than the concentration of the feed solution (5 mM). Therefore, the rejection of the membrane toward Sc3+ is not due to the adsorption of ions to NDNG in this work.
Figure 5.
Theoretical computations of the interaction energy between REEs and NDNG
(A) The most stable optimized geometries and the corresponding electrostatic potential surfaces of N6 and N5 on the sheets of NDNG and nitrate of Sc, La, Eu. The purple region represents negative ESP, while the green region represents positive ESP.
(B) Comparison of the interaction energies of the REEs (Sc, La, Eu) with N6 and N5.
(C) Upper panel: the most stable optimized geometries and the corresponding electrostatic potential surfaces of the complexes of REEs with N6 and N5 along with their interaction energies, respectively, where the cations are Sc3+, La3+, and Eu3+. Lower panel: corresponding color-filled maps of electron location function.
Conclusions
In summary, we have developed a one-step route for synthesizing nitrogen-doped nanopores graphene and clarified clearly the mechanism of multiple confinement combustion strategy. Because of steric hindrance resulting from interspace of formed Zn-hydrotalcite, planar N include pyrrolic-N and pyridinic-N were generated selectively in the flat 2D nanoreactor in combustion. Meanwhile, the N doping amount and pore size of the synthetic NDNG material can be tuned by adjusting the amount of amino acid and salt, respectively. The as-prepared NDNG membrane showed long-term stability and excellent separation performance toward REEs. Theoretical simulations also revealed that planar N, especially pyrrolic-N, served as selective adsorption sites during the separation process. Combining the simple and efficient preparation approach and excellent separation stability, we conclude that fabricating NDNG membranes is a promising membrane strategy for green separation of REEs. In addition, this novel synthetic method can be extended to the synthesis of other heteroatom-doped nanopores materials, which will have great potentials in not only the membrane separation field but also in other fields such as catalysis, sensors, and supercapacitors.
Limitations of the study
In addition to the NDNG being prepared in this study, more heteroatoms doping porous 2D materials can be prepared and applied for more potential breakthroughs in structure control and performance enhancement.
Resource availability
Lead contact
Hongdeng Qiu (hdqiu@licp.cas.cn) serves as the lead contact.
Materials availability
No new unique reagents were generated.
Data and code availability
Data supporting the main conclusions of this study are available from the corresponding author upon request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the National Key R&D Program of China (2019YFC1905501), the National Natural Science Foundation of China (No. 21974146, 21822407, 21675164), CAS “Light of West China” Program and the Foundation for Sci & Tech Research Project of Gansu Province (18JR3RA387).
Author contributions
H.T., Z.L., and H.Q. conceived the project. Z.L. and H.Q. supervised the project. H.T. and Z.L. performed the experiments and characterizations. X.Z. and S.C. carried out the theoretical simulations and analysis. H.T., Z.L., and Q.L. carried out AFM testing and analyzed the data. H.T., J.W., and Y.Y. analyzed the ICP-MS data. H.T., Z.L., J. C. and H.Q. co-wrote the manuscript. All authors discussed the results and commented on the manuscript.
Declaration of interests
A China patent application (202010861481.0) has been filed, with H.Q., H.T., Z.L., and J.C. as inventors, covering the method for fabricating nitrogen-doped nanoporous graphene membranes described herein. The authors declare no competing interests.
Published: January 22, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101920.
Contributor Information
Zhan Li, Email: lizhancg@licp.cas.cn.
Hongdeng Qiu, Email: hdqiu@licp.cas.cn.
Supplemental information
References
- Ahn H., Kim T., Choi H., Yoon C., Um K., Nam J., Ahn K.H., Lee K. Gelation of graphene oxides induced by different types of amino acids. Carbon. 2014;71:229–237. [Google Scholar]
- Ai W., Jiang J., Zhu J., Fan Z., Wang Y., Zhang H., Huang W., Yu T. Supramolecular polymerization promoted in situ fabrication of nitrogen-doped porous graphene sheets as anode materials for Li-Ion Batteries. Adv. Energy Mater. 2015;5:1500559. [Google Scholar]
- Algara-Siller G., Lehtinen O., Wang F.C., Nair R.R., Kaiser U., Wu H.A., Geim A.K., Grigorieva I.V. Square ice in graphene nanocapillaries. Nature. 2015;519:443–445. doi: 10.1038/nature14295. [DOI] [PubMed] [Google Scholar]
- Anitha M., Ambare D.N., Kotekar M.K., Singh D.K., Singh H. Studies on Permeation of Nd (III) through supported liquid membrane using DNPPA plus TOPO as Carrier. Sep. Sci. Technol. 2013;48:2196–2203. [Google Scholar]
- Bian Y., Wang H., Hu J., Liu B., Liu D., Dai L. Nitrogen-rich holey graphene for efficient oxygen reduction reaction. Carbon. 2020;162:66–73. [Google Scholar]
- Brigham D.M., Ivanov A.S., Moyer B.A., Delmau L.H., Bryantsev V.S., Ellis R.J. Trefoil-shaped outer-sphere ion clusters mediate lanthanide(III) ion transport with diglycolamide ligands. J. Am. Chem. Soc. 2017;139:17350–17358. doi: 10.1021/jacs.7b07318. [DOI] [PubMed] [Google Scholar]
- Cheisson T., Schelter E.J. Rare earth elements: mendeleev's bane, modern marvels. Science. 2019;363:489–493. doi: 10.1126/science.aau7628. [DOI] [PubMed] [Google Scholar]
- Chen L., Shi G., Shen J., Peng B., Zhang B., Wang Y., Bian F., Wang J., Li D., Qian Z. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature. 2017;550:415–418. doi: 10.1038/nature24044. [DOI] [PubMed] [Google Scholar]
- Chen L., Wu Y., Dong H., Meng M., Li C., Yan Y., Chen J. An overview on membrane strategies for rare earths extraction and separation. Sep. Purif. Technol. 2018;197:70–85. [Google Scholar]
- Cohen-Tanugi D., Grossman J.C. Water desalination across nanoporous graphene. Nano Lett. 2012;12:3602–3608. doi: 10.1021/nl3012853. [DOI] [PubMed] [Google Scholar]
- Croft C.F., Almeida M., Cattrall R.W., Kolev S.D. Separation of lanthanum(III), gadolinium(III) and ytterbium(III) from sulfuric acid solutions by using a polymer inclusion membrane. J. Membr. Sci. 2018;545:259–265. [Google Scholar]
- Cui K., Gao B., Tai M., Su B. A facile bionic strategy towards Gd (III)-imprinted membranes via interlaced stacking of one-dimensional/two-dimensional nanocomposite materials. J. Taiwan Inst. Chem. Eng. 2019;95:652–659. [Google Scholar]
- Dimiev A., Tour J. Mechanism of graphene oxide formation. ACS Nano. 2014;8:3060–3068. doi: 10.1021/nn500606a. [DOI] [PubMed] [Google Scholar]
- Ding W., Wei Z., Chen S., Qi X., Yang T., Hu J., Wang D., Wan L.-J., Alvi S., Li L. Space-confinement-Induced synthesis of pyridinic- and pyrrolic-nitrogen-doped graphene for the catalysis of oxygen reduction. Angew. Chem. Int. Ed. 2013;52:11755–11759. doi: 10.1002/anie.201303924. [DOI] [PubMed] [Google Scholar]
- Du D., Li P., Ouyang J. Nitrogen-doped reduced graphene oxide prepared by simultaneous thermal reduction and nitrogen doping of graphene oxide in air and its application as an electrocatalyst. ACS Appl. Mater. Interfaces. 2015;7:26952–26958. doi: 10.1021/acsami.5b07757. [DOI] [PubMed] [Google Scholar]
- Fang Q., Zhou X., Deng W., Zheng Z., Liu Z. Freestanding bacterial cellulose-graphene oxide composite membranes with high mechanical strength for selective ion permeation. Sci. Rep. 2016;6:33185. doi: 10.1038/srep33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florek J., Giret S., Juere E., Lariviere D., Kleitz F. Functionalization of mesoporous materials for lanthanide and actinide extraction. Dalton Trans. 2016;45:14832–14854. doi: 10.1039/c6dt00474a. [DOI] [PubMed] [Google Scholar]
- Garcia-Gallastegui A., Iruretagoyena D., Gouvea V., Mokhtar M., Asiri A.M., Basahel S.N., Al-Thabaiti S.A., Alyoubi A.O., Chadwick D., Shaffer M.S.P. Graphene oxide as support for layered double hydroxides: enhancing the CO2 adsorption capacity. Chem. Mater. 2012;24:4531–4539. [Google Scholar]
- Giret S., Hu Y., Masoumifard N., Boulanger J.-F., Juere E., Kleitz F., Lariyiere D. Selective separation and preconcentration of scandium with mesoporous silica. ACS Appl. Mater. Interfaces. 2018;10:448–457. doi: 10.1021/acsami.7b13336. [DOI] [PubMed] [Google Scholar]
- Hong G., Shen L., Wang M., Yang Y., Wang X., Zhu M., Hsiao B. Nanofibrous polydopamine complex membranes for adsorption of Lanthanum (III) ions. Chem. Eng. J. 2014;244:307–316. [Google Scholar]
- Hu Y., Florek J., Lariviere D., Fontaine F.-G., Kleitz F. Recent advances in the separation of rare earth elements using mesoporous hybrid materials. Chem. Rec. 2018;18:1261–1276. doi: 10.1002/tcr.201800012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Mao Y., Ying Y., Liu Y., Sun L., Peng X. Salt concentration, pH and pressure controlled separation of small molecules through lamellar graphene oxide membranes. Chem. Commun. (Camb.) 2013;49:5963–5965. doi: 10.1039/c3cc41953c. [DOI] [PubMed] [Google Scholar]
- Huang K., Liu G., Lou Y., Dong Z., Shen J., Jin W. A graphene oxide membrane with highly selective molecular separation of aqueous organic solution. Angew. Chem. Int. Ed. 2014;53:6929–6932. doi: 10.1002/anie.201401061. [DOI] [PubMed] [Google Scholar]
- Joshi R.K., Carbone P., Wang F., Kravets V.G., Su Y., Grigorieva I.V., Wu H., Geim A.K., Nair R.R. Precise and ultrafast molecular sieving through graphene oxide membranes. Science. 2014;343:752–754. doi: 10.1126/science.1245711. [DOI] [PubMed] [Google Scholar]
- Kim D., Powell L., Delmau L.H., Peterson E.S., Herchenroeder J., Bhave R.R. A supported liquid membrane system for the selective recovery of rare earth elements from neodymium-based permanent magnets. Sep. Sci. Technol. 2016;51:1716–1726. [Google Scholar]
- Krebs E., Grabill L., Riemann A. Amino acid nanopatterning on graphite. Surf. Sci. 2018;678:143–148. [Google Scholar]
- Li J., Lin L., Rui D., Li Q., Zhang J., Kang N., Zhang Y., Peng H., Liu Z., Xu H. Electron hole symmetry breaking in charge transport in nitrogen-doped graphene. ACS Nano. 2017;11:4641–4650. doi: 10.1021/acsnano.7b00313. [DOI] [PubMed] [Google Scholar]
- Li K., Gao Q., Yadavalli G., Shen X., Lei H., Han B., Xia K., Zhou C. Selective adsorption of gd3+ on a magnetically retrievable imprinted chitosan/carbon nanotube composite with high capacity. ACS Appl. Mater. Interfaces. 2015;7:21047–21055. doi: 10.1021/acsami.5b07560. [DOI] [PubMed] [Google Scholar]
- Li Z., Liu Y., Zhao Y., Zhang X., Qian L., Tian L., Bai J., Qi W., Yao H., Gao B. Selective separation of metal ions via monolayer nanoporous graphene with carboxyl groups. Anal. Chem. 2016;88:10002–10010. doi: 10.1021/acs.analchem.6b02175. [DOI] [PubMed] [Google Scholar]
- Li Z., Zhang X., Tan H., Qi W., Wang L., Ali M., Zhang H., Chen J., Hu P., Fan C. Combustion fabrication of nanoporous graphene for ionic separation membranes. Adv. Funct. Mater. 2018;28:1805026. [Google Scholar]
- Lin Z., Waller G., Liu Y., Liu M., Wong C. Facile synthesis of nitrogen-doped graphene via pyrolysis of graphene oxide and urea, and its electrocatalytic activity toward the oxygen-reduction reaction. Adv. Energy Mater. 2012;2:884–888. [Google Scholar]
- Lipton J., Weng G.-M., Rӧhr J.A., Wang H., Taylor A.D. Layer-by-layer assembly of two-dimensional materials: meticulous control on the nanoscale. Matter. 2020;2:1148–1165. [Google Scholar]
- Liu E., Xu X., Zheng X., Zhang F., Liu E., Li C. An ion imprinted macroporous chitosan membrane for efficiently selective adsorption of dysprosium. Sep. Purif. Technol. 2017;189:288–295. [Google Scholar]
- Liu Y., Tian S., Meng X., Dai X., Liu Z., Meng M., Han J., Wang Y., Chen R., Yan Y. Synthesis, characterization, and adsorption properties of a Ce(III)-imprinted polymer supported by mesoporous SBA-15 matrix by a surface molecular imprinting technique. Can. J. Chem. 2014;92:257–266. [Google Scholar]
- Lu J., Wu Y., Lin X., Gao J., Dong H., Chen L., Qin Y., Wang L., Yan Y. Anti-fouling and thermosensitive ion-imprinted nanocomposite membranes based on grapheme oxide and silicon dioxide for selectively separating europium ions. J. Hazard. Mater. 2018;353:244–253. doi: 10.1016/j.jhazmat.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Men B., Sun Y., Li M., Hu C., Zhang M., Wang L., Tang Y., Chen Y., Wan P., Pan J. Hierarchical metal-free nitrogen-doped porous graphene/carbon composites as an efficient oxygen reduction reaction catalyst. ACS Appl. Mater. Inter. 2016;8:1415–1423. doi: 10.1021/acsami.5b10642. [DOI] [PubMed] [Google Scholar]
- Meng C., Sheng Y., Chen Q., Tan H., Liu H. Exceptional chiral separation of amino acid modified graphene oxide membranes with high-flux. J. Membr. Sci. 2017;526:25–31. [Google Scholar]
- Pawlak R., Liu X., Ninova S., D’Astolfo P., Drechsel C., Sangtarash S., Häner R., Decurtins S., Sadeghi H., Lambert C.J. Bottom-up synthesis of nitrogen-doped porous graphene nanoribbons. J. Am. Chem. Soc. 2020;142:12568–12573. doi: 10.1021/jacs.0c03946. [DOI] [PubMed] [Google Scholar]
- Pei L., Wang L., Yu G. Separation of Eu(III) with supported dispersion liquid membrane system containing D2EHPA as carrier and HNO3 solution as stripping solution. J. Rare Earths. 2011;29:7–14. [Google Scholar]
- Seo D.H., Pineda S., Woo Y.C., Xie M., Murdock A.T., Ang E.Y.M., Jiao Y., Park M.J., Lim S.I., Lawn M. Anti-fouling graphene-based membranes for effective water desalination. Nat. Commun. 2018;9:683. doi: 10.1038/s41467-018-02871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sint K., Wang B., Kral P. Selective ion passage through functionalized graphene nanopores. J. Am. Chem. Soc. 2008;130:16448–16449. doi: 10.1021/ja804409f. [DOI] [PubMed] [Google Scholar]
- Song L., Zhang H., Cai T., Chen J., Li Z., Guan M., Qiu H. Porous graphene decorated silica as a new stationary phase for separation of sulfanilamide compounds in hydrophilic interaction chromatography. Chin. Chem. Lett. 2019;30:863–866. [Google Scholar]
- Sun P., Zhu M., Wang K., Zhong M., Wei J., Wu D., Xu Z., Zhu H. Selective Ion penetration of graphene oxide membranes. ACS Nano. 2013;7:428–437. doi: 10.1021/nn304471w. [DOI] [PubMed] [Google Scholar]
- Tan H., Liu T., Zhang X., Shan Q., Chen J., Li Z., Ihara H., Qiu H. Preparation of vortex porous graphene chiral membrane for enantioselective separation. Anal. Chem. 2020;92:13630–13633. doi: 10.1021/acs.analchem.0c02446. [DOI] [PubMed] [Google Scholar]
- Tan H., Zhang X., Li Z., Qiu H. Small-scale nanoparticles pyrolyzed from layered hydrotalcite between graphene interlayers as intermediates to self-assemble into metal oxide nanosheets and hollow nanospheres. Chem. Nano. Mat. 2020;6:1270–1275. [Google Scholar]
- Thebo K.H., Qian X., Zhang Q., Chen L., Cheng H.-M., Ren W. Highly stable graphene-oxide-based membranes with superior permeability. Nat. Commun. 2018;9:1486. doi: 10.1038/s41467-018-03919-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Wang L., Wu D., Xia W., Zhao H., Jia D. Hydrothermal synthesis of nitrogen-doped graphene hydrogels using amino acids with different acidities as doping agents. J. Mater. Chem. A. 2014;2:8352–8361. [Google Scholar]
- Wang Z., Brown A.T., Tan K., Chabal Y.J., Balkus K.J., Jr. Selective extraction of thorium from rare earth elements using wrinkled mesoporous carbon. J. Am. Chem. Soc. 2018;140:14735–14739. doi: 10.1021/jacs.8b07610. [DOI] [PubMed] [Google Scholar]
- Werber J.R., Osuji C.O., Elimelech M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016;1:16018. [Google Scholar]
- Wu D., Song W., Chen L., Duan X., Xia Q., Fan X., Li Y., Zhang F., Peng W., Wang S. High-performance porous graphene from synergetic nitrogen doping and physical activation for advanced nonradical oxidation. J. Hazard. Mater. 2020;381:121010. doi: 10.1016/j.jhazmat.2019.121010. [DOI] [PubMed] [Google Scholar]
- Xu J., Wang K., Zu S., Han B., Wei Z. Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets with synergistic effect for energy storage. ACS Nano. 2010;4:5019–5026. doi: 10.1021/nn1006539. [DOI] [PubMed] [Google Scholar]
- Yan Y., Li W., Kral P. Enantioselective molecular transport in multilayer graphene nanopores. Nano Lett. 2017;17:6742–6746. doi: 10.1021/acs.nanolett.7b02846. [DOI] [PubMed] [Google Scholar]
- Yusoff M.M., Mostapa N.R.N., Sarkar M.S., Biswas T.K., Rahman M.L., Arshad S.E., Sarjadi M.S., Kulkarni A.D. Synthesis of ion imprinted polymers for selective recognition and separation of rare earth metals. J. Rare Earths. 2017;35:177–186. [Google Scholar]
- Zheng X., Zhang Y., Zhang F., Li Z., Yan Y. Dual-template docking oriented ionic imprinted bilayer mesoporous films with efficient recovery of neodymium and dysprosium. J. Hazard. Mater. 2018;353:496–504. doi: 10.1016/j.jhazmat.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Zheng X., Zhang F., Liu E., Xu X., Yan Y. Efficient recovery of neodymium in acidic system by free-standing dual-template docking oriented ionic imprinted mesoporous films. ACS Appl. Mater. Interfaces. 2017;9:730–739. doi: 10.1021/acsami.6b13049. [DOI] [PubMed] [Google Scholar]
- Zhou D., Cui Y., Xiao P., Jiang M., Han B. A general and scalable synthesis approach to porous graphene. Nat. Commun. 2014;5:4716. doi: 10.1038/ncomms5716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the main conclusions of this study are available from the corresponding author upon request.