Abstract
Background
Methylphenidate, modafinil, and amantadine are commonly prescribed medications for alleviating fatigue in multiple sclerosis (MS); however, the evidence supporting their efficacy is sparse and conflicting. Our goal was to compare the efficacy of these three medications against each other and placebo in patients with MS-related fatigue.
Methods
In this randomized, double-blind, placebo-controlled, four-sequence, four-period crossover trial, patients with MS who reported fatigue and had a Modified Fatigue Impact Scale (MFIS) score of more than 33 were recruited at two academic MS centers in the US. Participants received oral amantadine (up to 100 mg twice daily), modafinil (up to 100 mg twice daily), methylphenidate (up to 10 mg twice daily), or placebo, each given for up to six weeks. All patients were to receive all four study medications, in turn, with two-week washout periods between medications. A biostatistician prepared a concealed allocation schedule, stratified by site, randomly assigning a sequence of medications, in blocks of eight, to a consecutive series of numbers. At the time of enrollment, study pharmacists assigned each participant to the next consecutive number (and hence the sequence of study medications). The statistician and pharmacists had no role in assessing the participants or collecting data, and the participants, caregivers, and assessors were blinded to allocation. The primary outcome measure was the MFIS measured while taking the highest tolerated dose at week five of each medication period, analyzed using a linear mixed-effect regression model. This trial is registered with ClinicalTrials.gov, NCT03185065.
Findings
Between October 4, 2017, and February 27, 2019, 141 patients were enrolled and randomly assigned to one of four medication administration sequences (35 patients to amantadine, placebo, modafinil, methylphenidate sequence; 34 patients to placebo, methylphenidate, amantadine, modafinil sequence; 35 patients to modafinil, amantadine, methylphenidate, placebo sequence; and 37 patients to methylphenidate, modafinil, placebo, amantadine sequence). Data from 136 participants were available for the intent-to-treat analysis of the primary outcome. The estimated mean values of MFIS total scores (95% CI) at baseline and the maximal tolerated dose were as follows: 51.3 (49.0 to 53.6) at baseline, 40.6 (38.2 to 43.1) with placebo, 41.3 (38.8 to 43.7) with amantadine, 39.0 (36.6 to 41.4) with modafinil, and 38.6 (36.2 to 41.0) with methylphenidate (P=0.20 for the overall medication effect in the linear mixed-effect regression model). As compared to placebo [38 patients (31%)], higher proportions of participants reported adverse events while taking amantadine [49 patients (39%)], modafinil [50 patients (40%)], and methylphenidate [51 patients (40%)]. Three serious adverse events (SAEs) occurred during the study (pulmonary embolism and myocarditis while taking amantadine and an MS exacerbation requiring hospitalization while taking modafinil).
Interpretation
Amantadine, modafinil, and methylphenidate were not superior to placebo in improving MS-related fatigue and caused more frequent adverse events. The results of this study do not support an indiscriminate use of amantadine, modafinil, and methylphenidate for the treatment of fatigue in MS.
Keywords: multiple sclerosis, fatigue, amantadine, modafinil, methylphenidate, randomized controlled trial
Introduction
Fatigue, defined as a subjective lack of physical or mental energy perceived by the individual with usual activities, is one of the most common and disabling symptoms of multiple sclerosis (MS) and affects more than 75% of patients at some point during their disease.1–3 Fatigue negatively affects the health-related quality of life and is described as the worst symptom of the disease by more than 50% of patients.4 Fatigue is a subjective symptom and is difficult (but important) to distinguish from excessive daytime sleepiness or motor and cognitive fatigability. Secondary causes, such as sleep disorders, thyroid dysfunction, and anaemia can contribute to fatigue severity. Comorbid depression is common among patients with MS fatigue5, and MS exacerbations may worsen chronic fatigue.6 Despite the high prevalence and major consequences of fatigue in MS, the relative contribution of various pathophysiological mechanisms (such as immune and endocrine abnormalities and structural and functional brain changes) remains unclear. Exercise and behavioral and pharmacological interventions have been used for the management of MS fatigue. There is evidence that exercise7 and cognitive-behavioral therapy,8 may be effective symptomatic treatments of MS-related fatigue. On the other hand, the evidence supporting the use of medications for the treatment of MS-related fatigue is minimal and conflicting.9 Although the US Food and Drug Administration and the European Medicines Agency have not approved any medication to treat fatigue in patients with MS, clinicians often prescribe various drugs off-label to help relieve this common and disabling symptom.
Amantadine, modafinil, and amphetamine-like stimulants (such as methylphenidate, amphetamine/dextroamphetamine, and lisdexamfetamine) are among the most commonly used medications to treat MS fatigue.10,11 Both amantadine12–15 and modafinil14,16–20 have been tested in several clinical trials for MS fatigue. Still, the methodological shortcomings and conflicting results have prevented any definite conclusion regarding their efficacy.21,22 Aside from three clinical trials of pemoline15,23,24, a stimulant that is no longer available in the United States, psychostimulants have not been tested in randomized controlled trials for MS fatigue. Methylphenidate has been tested in several randomized controlled trials of fatigue in conditions other than MS25,26 that have yielded conflicting results. Most of these medications are wake-promoting agents; however, MS fatigue is not equivalent to excessive daytime sleepiness. Despite the widespread use of amantadine, modafinil, and amphetamine-like stimulants, it remains unclear if any improves MS-related fatigue better than placebo, and if so, which one is most effective, better tolerated, and has the fewest side effects. It is also unknown whether comorbid conditions such as depression or potentially relevant factors, such as MS subtype, the severity of the physical disability, or the use of immune-based disease-modifying therapies alter the effect of these medications on MS fatigue. In a pragmatic randomized trial, we compared the efficacy, safety, and tolerability of amantadine, modafinil, methylphenidate, and placebo in patients with MS-related fatigue.
Methods
Study Design
Treatment of Fatigue with Methylphenidate, Modafinil and Amantadine in MS (TRIUMPHANT-MS) was a pragmatic randomized, crossover, four-sequence, four-period, double-blind (participants and investigators), two-center trial of three commonly used medications for the treatment of MS-related fatigue (amantadine, modafinil, methylphenidate) versus placebo in patients with MS and fatigue. Using a balanced Latin square crossover design, subjects were allocated, in a double-blind, randomized fashion, to one of the four treatment sequences (Appendix page 7, Figure S1): A) amantadine, placebo, modafinil, methylphenidate; B) placebo, methylphenidate, amantadine, modafinil; C) modafinil, amantadine, methylphenidate, placebo; and D) methylphenidate, modafinil, placebo, and amantadine. Each treatment period lasted six weeks, and there was a two-week washout period between each treatment period. The two-week washout period was selected based on the elimination half-life of study medications27–29 and the clinically-observed putative anti-fatigue effects (which quickly disappear after stopping the medications).
The trial was funded by the Patient-Centered Outcome Research Institute (PCORI) (ClinicalTrials.gov number, NCT03185065). The authors designed the study and performed data collection and analysis. A stakeholder advisory committee comprised of academic and community neurologists, experts in MS fatigue, patients with MS, and a representative from the National MS Society provided guidance and feedback during the design, execution, and reporting of the trial results. A data and safety monitoring board (DSMB) oversaw the adverse events reported during the trial and provided guidance regarding the safety of the trial and its continuation. The DSMB was independent of the rest of the study, did not have access to the efficacy data, and there was no plan for interim efficacy analysis.
The trial was exempt from the requirements for an Investigational New Drug application with the FDA. The institutional review boards approved the study at Johns Hopkins University (JHU) and the University of California, San Francisco (UCSF) (approval number at JHU: IRB00119702, approval number at UCSF: 17–22584). All participants provided written informed consent.
Participants
Participants were recruited through physicians and clinic referrals, and via advertisement at two academic specialty MS centers (JHU and UCSF MS Clinics). All participants gave written informed consent before any study procedure. Patients were eligible for participation in this study if they were 18 years of age or older, had a diagnosis of MS (according to the 2010 McDonald criteria)30, reported fatigue as a symptom, and had a screening Modified Fatigue Impact Scale (MFIS) score > 33, had an Expanded Disability Status Scale (EDSS) score at the time of screening 0.0 to 7.0 (inclusive) and were not on any medication for the treatment of fatigue (including the study medications) for at least two weeks before the screening visit. Exclusion criteria are shown in Table S1 (Supplement page 10).
Randomization and masking
Using the built-in random number generator in Stata (version 15, StataCorp LLC. College Station, TX, USA) a biostatistician at UCSF (JC) prepared a concealed allocation schedule, stratified by site, randomly assigning the four sequences, in blocks of eight, to a consecutive series of numbers. At the time of enrollment, the study pharmacist at each site assigned each participant the next consecutive number (and hence the sequence of study medications). Participants were randomly assigned to one of the above-mentioned treatment sequences in approximately a 1:1:1:1 ratio. Only the statistician building the allocation sequence and the pharmacists at each study site were aware of the study medication allocation sequence (i.e., were unblinded). All other study personnel remained blinded to the allocation sequence until the study database lock. Participants were also blinded to their allocation sequence while actively participating in the study.
Procedures
The screening visit was the only in-person study visit. Screening visit procedures are outlined in the Supplement (page 2). The baseline values of the MFIS, Quality of Life in Neurological Disorders (NeuroQoL) fatigue item bank, and Epworth Sleepiness Scale (ESS) were obtained through remote (web-based) answering within three days of initiation of the first medication period. The study endpoints were measured during the fifth week of each study period (while the participants were taking the highest or maximally tolerated dose of each study medication). All efficacy measures were patient-reported outcomes that were administered remotely (web-based). After completing a medication period, the next assigned medication was mailed to participants. The next-period medication was initiated after at least two weeks of washout from the prior period medication.
Study medications were compounded, bottled, labeled, and shipped to each study site pharmacy by the University of Iowa Pharmaceuticals. The pharmacist at each site (who had the randomization table provided by the statistician) dispensed the study medication to the study coordinator based on the participant’s randomization number. Study personnel and participants remained blinded during all the study evaluations.
During each study period, participants received a bottle containing red-colored capsules and a bottle containing blue-colored capsules. This plan was designed to keep the titration schedule for all treatment periods the same and maintain participants and personnel blinding to treatment assignments. Study medications were titrated over six weeks, according to Figure S2 (Supplement page 8). The maximum dose of each medication taken during weeks four and five was 100 mg twice daily for amantadine, 100 mg twice daily for modafinil, and 10 mg twice daily for methylphenidate. The maximum doses of amantadine12–15 and modafinil14,16–20 were selected based on the most common doses used in previous clinical trials. Because there was no previous clinical trial of methylphenidate in MS fatigue, we selected the maximum dose based on the average dose of methylphenidate that was reported to be efficacious in a study of fatigue in patients with advanced illnesses.25,26 Details of study medication titration and data collection are presented in the Supplement page 3 and page 4.
Outcomes
The primary outcome of the study was the MFIS total score measured during the fifth week of each treatment period. The MFIS is a validated questionnaire with 21 items and assesses different dimensions of fatigue, including: physical, cognitive, and psychosocial.31 The total score is the sum of the scores for each of the 21 items, and a higher score indicates more severe fatigue (minimum of 0 and a maximum of 84). Minimally important difference (MID) in the MFIS score change in MS ranged from 4 to 8.32
Secondary endpoints were scores on the Neuro-QoL fatigue item bank33 and the ESS, a measure of daytime sleepiness34. These outcomes were measured at the same time as the MFIS. Post hoc exploratory outcomes included the physical, cognitive, and psychosocial MFIS subscores and the response to the following question: “Taking into consideration the possible benefits and/or disadvantages of this medication, would you choose it, going forward to treat your MS fatigue?”.
Adverse events and tolerability data were collected by contacting the participants once a week via phone calls, emails, or text messages.
Statistical analysis
At the time this trial was designed, the only available report about the clinical relevance of a change in MFIS total score was a study by Kos et al., which considered a 10 point or more change in the MFIS score to be clinically relevant.35 Based on longitudinal measurements of MFIS total scores during a previous clinical trial36, we found a between-subject variance of 330 and within-subject variance of 80 with an intra-class correlation (ICC) of 0.80 (95% confidence interval of 0.70 to 0.88) in the MFIS total score. A sample size of 91 patients (364 data points) would provide the trial with 90% power, at a two-sided significance level of 5% (Bonferroni corrected for 6 pairwise comparisons), to detect at least a 10-point difference in MFIS scores between the placebo and medication groups. We anticipated a dropout rate of 20% in each medication period, and as such, a sample size of 136 would provide the required number of data points.
The efficacy dataset included all participants who had the primary outcome measured in week 5 of at least one treatment period. Following the intention-to-treat (ITT) principle, participants were analyzed according to the randomized sequence assignment. The safety dataset included all patients who received at least one dose of any study medication. Safety was analyzed according to the actual treatment received.
We used a linear mixed-effect regression model in the efficacy dataset for the primary outcome measure (MFIS total score) utilizing restricted maximum likelihood fitting, an exchangeable covariance structure and Kenward-Roger degree of freedom adjustments. The fixed predictors were study medications (categorical placebo, amantadine, modafinil, methylphenidate), treatment sequence (categorical 1 to 4), treatment period (categorical 1 to 4), a baseline measure of the outcome and study site (categorical JHU/UCSF); subjects were the random effect. If the adjusted test of treatment differences was significant at the 0.05 level, we would make pairwise comparisons between study treatments using estimated contrasts at the 0.05 level. If the adjusted test of treatment differences was not significant, the pairwise comparisons were considered exploratory. Estimated mean values, along with standard errors, were reported for each drug and the placebo. Other efficacy outcomes were analyzed using similar mixed-effect models. The use of the mixed-effect regression model provided protection against bias due to missing data. The statistical analyses were done by independent faculty (CM and CJ), using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Sensitivity, exploratory, and heterogeneity of treatment effects analyses are presented in Supplement page 5.
The assessment of safety was based on the frequency of adverse events. Tolerability was reported as the proportion of participants who achieved maximum dose and half-maximum dose or discontinued the medication before the end of the treatment period. We also calculated the estimated average dose and 95% confidence intervals by treating the maximum dose as the outcome in a mixed model analysis (similar to the primary outcome analysis) with a single predictor of medication and with participants as a random effect.
Role of the funding source
The funder had no role in study design, data collection, analysis, and interpretation, or writing of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
One hundred sixty-nine patients with MS were screened for participation in this clinical trial. Of these, 146 were eligible, and between October 4, 2017, and February 27, 2019, 141 underwent randomization. One hundred eleven participants completed all four medication periods (Figure 1). The demographic and baseline characteristics (at the time of screening) of randomized participants are shown in Table 1. The demographic and baseline characteristics (at the time of screening) of participants who completed all four medication periods are shown in Table S2 (Supplement page 11).
Figure 1-.
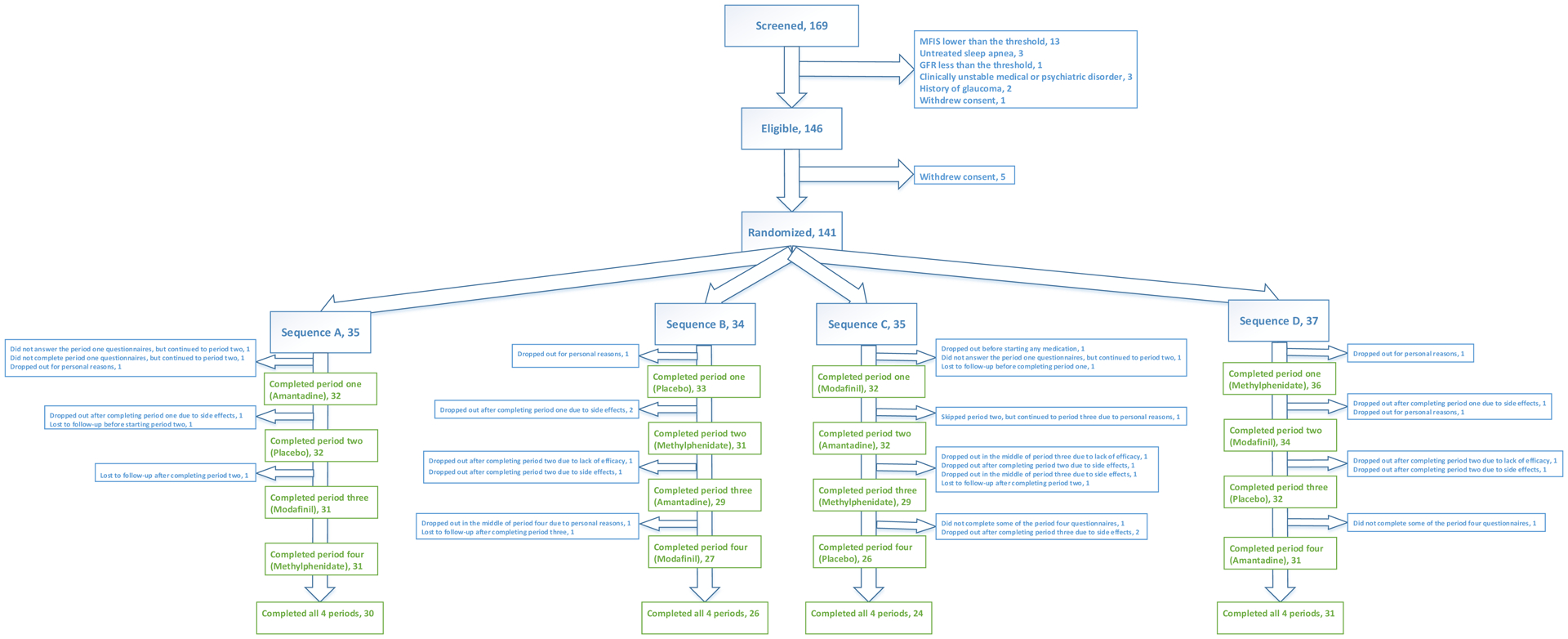
Trial profile
Sequence A: Amantadine-Placebo-Modafinil-Methylphenidate; Sequence B: Placebo-Methylphenidate-Amantadine-Modafinil; Sequence C: Modafinil-Amantadine-Methylphenidate-Placebo; Sequence D: Methylphenidate-Modafinil-Placebo-Amantadine
Table 1:
Baseline characteristics of randomized participants at the time of screening
| Variable | All participants (N=141) |
Sequence A (N=35) |
Sequence B (N=34) |
Sequence C (N=35) |
Sequence D (N=37) |
|---|---|---|---|---|---|
| Age (Mean [SD]) | 46.8 ± 10.7 | 48.3 [9.9] | 46.9 [12.4] | 46.1[10.3] | 45.8 [10.2] |
| Female | 109 (77%) | 27 (77%) | 26 (76%) | 27 (77%) | 29 (78%) |
| Race | |||||
| White | 107 (76%) | 27 (77%) | 25 (74%) | 26 (74%) | 29 (78%) |
| African American | 19 (13.5%) | 4 (11%) | 6 (18%) | 5 (14%) | 4 (11%) |
| Other | 15 (11%) | 4 (11%) | 3 (9%) | 4 (11%) | 4 (11%) |
| Ethnicity | |||||
| Hispanic | 15 (11%) | 6 (17%) | 3 (9%) | 2 (6%) | 4 (11%) |
| Non-Hispanic | 126 (89%) | 29 (83%) | 31 (91%) | 33 (94%) | 33 (89%) |
| MS subtype | |||||
| Relapsing-remitting | 106 (75%) | 29 (83%) | 27 (79%) | 25 (71%) | 25 (68%) |
| Secondary progressive | 19 (14%) | 2 (6%) | 5 (15%) | 5 (14%) | 7 (19%) |
| Primary progressive | 15 (11%) | 4 (11%) | 2 (6%) | 4 (11%) | 5 (14%) |
| Unknown | 1 (1%) | 0 (0) | 0 (0) | 1 (3%) | 0 (0) |
| Taking a DMT at the time of screening | 112 (79%) | 27 (77%) | 30 (88%) | 25 (71%) | 30 (81%) |
| EDSS Score (Median [IQR]) | 3.0 [2.0–4.5] | 2.5 [2.0 −4.0] | 3.0 [2.0–4.0] | 3.0 [2.0–4.5] | 3.0 [2.0–5.0] |
| HADS Depression-subscale score (Mean [SD]) | 5.5 ± 3.3 | 6.3 [3.4] | 5.2 [2.8] | 4.7 [2.8] | 5.9 [3.5] |
| MFIS Total Score (Mean [SD]) | 53.9 ±11.4 | 57.7 [11.7] | 54.9 [12.0] | 51.4 [10.1] | 51.8 [11.2] |
| MFIS physical subscale Score (Mean [SD]) | 25.3 ± 5.9 | 26.1 [6.1] | 25.8 [6.0] | 25.1 [5.5] | 24.2 [6.0] |
| MFIS cognitive subscale score (Mean [SD]) | 23.7 ± 7.2 | 26.2 [7.3] | 24.0 [7.2] | 21.4 [7.1] | 23.1 [6.8] |
| MFIS psychosocial subscale score (Mean [SD]) | 4.9 ± 1.8 | 5.4 [1.7] | 5.1 [2.1] | 4.8 [1.6] | 4.4 [1.8] |
| ESS score (Mean [SD]) | 10.5 ± 5.0 | 11.6 [5.3] | 8.8 [5.5] | 11.5 [4.3] | 10.0 [4.5] |
| NeuroQoL Fatigue Item Bank T-score (Mean [SD]) | 58.4 ± 5.9 | 59.3 [6.4] | 59.5 [6.4] | 57.9 [5.4] | 56.9 [5.3] |
MS: multiple sclerosis, DMT: disease-modifying therapy, EDSS: Expanded Disability Status Scale, ESS: Epworth Sleepiness Scale, HADS: Hospital Anxiety and Depression Scale, MFIS: Modified Fatigue Impact Scale, NeuroQoL: Quality of Life in Neurological Disorders
Sequence A: Amantadine-Placebo-Modafinil-Methylphenidate; Sequence B: Placebo-Methylphenidate-Amantadine-Modafinil; Sequence C: Modafinil-Amantadine-Methylphenidate-Placebo; Sequence D: Methylphenidate-Modafinil-Placebo-Amantadine
There was a statistically significant association between the MFIS total score at the time of screening and the HADS Depression subscale score (Spearman’s rho=0.39, p-value<0.001) and the ESS score (Spearman’s rho=0.17, p-value=0.049), but not with the EDSS score (Spearman’s rho=0.14, p-value=0.097).
One hundred thirty-six participants completed at least one medication period and participated in the intention-to-treat efficacy analysis. The estimated mean values for the MFIS total score (95% CI) at the highest or maximum tolerated dose (week 5) in each medication period were as follows: 40.6 (38.2 to 43.1) with placebo, 41.3 (38.8 to 43.7) with amantadine, 39.0 (36.6 to 41.4) with modafinil, and 38.6 (36.2 to 41.0) with methylphenidate (Table 2). The primary analysis (the linear mixed-effect regression model) did not show any significant main effect of treatment (p=0.20), treatment sequence (p=0.44), treatment period (p=0.50) or site (p=0.60). The estimated means of MFIS total score did not differ among amantadine, modafinil, methylphenidate, and placebo (Table S3, Supplement page 12). Prespecified factors for analysis of heterogeneity of treatment effects, including relapsing-remitting versus progressive MS, high versus low screening depression scores, taking versus not-taking a disease-modifying therapy, and high versus low EDSS at the time of screening did not modify the effect of treatment on the MFIS total score (i.e., there was no heterogeneity of treatment effect based on these prespecified factors).
Table 2:
Mean baseline values and estimated means (95% CI) for each medication period
| Outcome | Measure | Mean Score | Estimated Mean Score | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Placebo (N=123) |
Amantadine (N=124) |
Modafinil (N=124) |
Methylphenidate (N=127) |
P-value for the overall medication effect$ | ||
| Prespecified primary | MFIS Total | 51.3 (49.0 to 53.6) | 40.6 (38.2 to 43.1) | 41.3 (38.8 to 43.7) | 39.0 (36.6 to 41.4) | 38.6 (36.2 to 41.0) | 0.20 |
| Prespecified secondary | NeuroQoL Fatigue T-score | 58.5 (57.6 to 59.4) | 53.1 (51.9 to 54.3) | 53.0 (51.7 to 54.2) | 52.5 (51.3 to 53.8) | 52.0 (50.8 to 53.2) | 0.42 |
| Prespecified secondary | ESS | 11.1 (10.2 to 11.9) | 9.4 (8.7 to 10.1) | 9.3 (8.6 to 10.1) | 8.3 (7.6 to9.1) | 8.8 (8.1 to 9.6) | 0.071 |
| Post hoc exploratory | MFIS physical subscale | 24.1 (22.9 to 25.3) | 18.9 (17.7 to 20.1) | 19.5 (18.3 to 20.6) | 18.4 (17.2 to 19.6) | 18.0 (16.9 to 19.2) | 0.21 |
| Post hoc exploratory | MFIS cognitive subscale | 22.4 (21.0 to 23.7) | 17.8 (16.6 to 19.0) | 18.0 (16.8 to 19.2) | 17.0 (15.8 to 18.2) | 17.2 (16.0 to 18.4) | 0.42 |
| Post hoc exploratory | MFIS psychosocial subscale | 4.8 (4.5 to 5.2) | 3.9 (3.6 to 4.2) | 3.9 (3.6 to 4.2) | 3.6 (3.3 to 3.9) | 3.4 (3.1 to 3.7) | 0.028 |
ESS: Epworth Sleepiness Scale, MFIS: Modified Fatigue Impact Scale, NeuroQoL: Quality of Life in Neurological Disorders
P-value for the overall medication effect in the mixed-effect regression model. The fixed predictors were study medications, treatment sequence, treatment period, the baseline level of the outcome, and the study site. Subjects were the random effect.
The average NeuroQoL fatigue item bank T- and ESS scores at baseline and at the maximally tolerated dose (week 5) of each study medication and pairwise comparisons with placebo are shown in Table 2 and Table S3 (Supplement page 12), respectively. There was no treatment effect on the average NeuroQoL fatigue item bank T-score (p=0.42), and the ESS score (p=0.071).
In post hoc exploratory analyses, we found no significant treatment effect on the physical and cognitive subscales of the MFIS. However, a significant treatment effect was seen on the psychosocial subscale (Table 2). On pairwise comparisons, methylphenidate improved the psychosocial subscale of the MFIS compared to placebo (adjusted mean difference −0.5, 95% CI: −0.8, −0.1) (Table S3, Supplement page 12).
Sensitivity analyses for carry-over effects were conducted by including an additional predictor of treatment in the previous time period in the mixed-effect models. There was no significant carry-over effect for the MFIS total score (p-values for the carry-over term: 0.66).
We evaluated the trial post hoc as a parallel-group design by restricting the primary efficacy outcome analysis to the first medication period. The estimated mean values for the MFIS total score (95% CI) at the highest or maximum tolerated dose (week 5) in the first medication period were as follows: 39.8 (35.1 to 44.5) with placebo, 43.4 (38.6 to 48.2) with amantadine, 37.2 (32.4 to 42.0) with modafinil, and 39.8 (35.3 to 44.4) with methylphenidate. There was no statistically significant difference among study drugs on the estimated mean values of MFIS total score in the first medication period (p-value for the overall medication effect: 0.34).
In a post hoc analysis of heterogeneity of treatment effect, there was an interaction between the baseline ESS score and treatment effect on the primary fatigue outcome (p-value of the interaction test=0.024). More than 50% of patients in the efficacy dataset had excessive daytime sleepiness (ESS score>10) at baseline. Among those, the estimated means of MFIS total scores for modafinil and methylphenidate were 4.1 points lower than the placebo (95%CI: −8.0, −0.3, and −7.9, −0.2, respectively) (Figure S3, Supplement page 9, and Table S4, Supplement page 14). In patients with no excessive daytime sleepiness at baseline (ESS score ≤10), the estimated means of MFIS total scores for amantadine, modafinil, and methylphenidate were not statistically significantly different from the placebo. The results of other post hoc exploratory analyses are presented in Appendix page 6.
Adverse events are shown in Table 3 and Table S5 (Supplement page 15). Thirty-eight participants (31%) reported at least one adverse event while taking placebo. Forty-nine (39%), 50 (40%), and 51 (40%) participants reported at least one adverse event while taking amantadine, modafinil, and methylphenidate, respectively. The total number of moderate-to-severe adverse events in each medication period was as follows: 28 with amantadine, 31 with modafinil, 40 with methylphenidate, and 18 with placebo. Three serious adverse events (SAEs) occurred during the study (pulmonary embolism and myocarditis while taking amantadine and an MS exacerbation requiring hospitalization while taking modafinil. None of these SAEs were judged by the principal investigators to be related to study medications.
Table 3.
Adverse events
| Placebo (n=124) |
Amantadine (n=127) |
Modafinil (n=125) |
Methylphenidate (n=129) |
|
|---|---|---|---|---|
| Adverse events (n) | 70 | 106 | 138 | 114 |
| Patients experiencing at least one adverse event | 38 (30.6%) | 49 (38.6%) | 50 (40.0%) | 51 (39.5%) |
| Cardiac disorders | 3 [3 (2.4%)] | 3 [3 (2.4%)] | 5 [2 (1.6%)] | 5 [4 (3.1%)] |
| Ear and labyrinth disorders | 0 [0] | 1 [1 (0.8%)] | 0 [0] | 0 [0] |
| Eye disorders | 0 [0] | 1 [1 (0.8%)] | 2 [2 (1.6%)] | 1 [1 (0.8%)] |
| Gastrointestinal disorders | 20 [10 (8.1%)] | 18 [14 (11.0%)] | 33 [19 (15.2%)] | 17 [13 (10.1%)] |
| General disorders and administration | 2 [2 (1.6%)] | 4 [4 (3.2%)] | 5 [5 (4.0%)] | 4 [4 (3.1%)] |
| Immune system disorders | 0 [0] | 1 [1 (0.8%)] | 1 [1 (0.8%)] | 0 [0] |
| Infections and infestations | 4 [3 (2.4%)] | 2 [2 (1.6%)] | 2 [2 (1.6%)] | 1 [1 (0.8%)] |
| Injury, poisoning, and procedural complications | 1 [1 (0.8%)] | 0 [0] | 0 [0] | 6 [4 (3.1%)] |
| Metabolism and nutrition disorders | 2 [2 (1.6%)] | 1 [1 (0.8%)] | 4 [3 (2.4%)] | 0 [0] |
| Musculoskeletal and connective tissue disorders | 5 [4 (3.2%)] | 1 [1 (0.8%)] | 2 [2 (1.6%)] | 1 [1 (0.8%)] |
| Nervous system disorders | 18 [13 (10.5%)] | 39 [24 (18.9%)] | 42 [22 (17.6%)] | 37 [20 (15.5%)] |
| Psychiatric disorders | 11 [10 (8.1%)] | 27 [20 (15.7%)] | 25 [18 (14.4%)] | 36 [23 (17.8%)] |
| Renal and urinary disorders | 0 [0] | 0 [0] | 1 [1 (0.8%)] | 0 [0] |
| Reproductive system | 1 [1 (0.8%)] | 1 [1 (0.8%)] | 2 [2 (1.6%)] | 2 [2 (1.6%)] |
| Respiratory disorders | 0 [0] | 2 [2 (1.6%)] | 2 [2 (1.6%)] | 2 [2 (1.6%)] |
| Skin and subcutaneous tissue disorders | 2 [2 (1.6%)] | 2 [2 (1.6%)] | 7 [5 (4.0%)] | 0 [0] |
| Vascular disorders | 1 [1 (0.8%)] | 3 [3 (2.4%)] | 5 [3 (2.4%)] | 2 [1 (0.8%)] |
| Serious adverse events | 0 [0] | 2 [2 (1.6%)] | 1 [1 (0.8%)] | 0 [0] |
Data are the number of adverse events in each System Organ Class [number of patients experiencing each type of event (% of cohort)
One hundred and seventeen (92%) participants tolerated the maximum dose of amantadine (200 mg daily), while 108 (86%) and 112 (87%) participants tolerated the maximum doses of modafinil (200 mg daily) and methylphenidate (20 mg daily), respectively. One hundred and seventeen (94%) participants tolerated the maximum number of placebo capsules. Seven (6%) participants discontinued amantadine before the end of the medication period, and 120 (95%) tolerated at least half of the maximum dose. Ten (8%) and seven (5%) participants stopped modafinil and methylphenidate before the end of the medication period, respectively. At least half of the maximum dose was tolerated by 115 (92%) participants for modafinil and 118 (92%) for methylphenidate.
The estimated means (95% CI) for the highest tolerated doses of medications were as follows: 186.4 (179.8 to 192.9) mg/day for amantadine, 178.3 (171.7 to 184.9) mg/day for modafinil and 17.5 (11.0 to 24.1) mg/day for methylphenidate.
Discussion
Our pragmatic, randomized, double-blind, crossover trial showed that amantadine, modafinil, and methylphenidate were not superior to placebo in improving MS-related fatigue measured with validated outcome measures. The average MFIS total score (on a scale from 0 to 84) improved by approximately 10 points on average from baseline in the placebo group, which is comparable to prior trials in MS fatigue.18 Compared to placebo, the MFIS score worsened by 0.7 points on average with amantadine, while it improved marginally with modafinil and methylphenidate (1.6 points and 2.0 points, respectively). These differences were not statistically nor clinically significant, as a minimum difference of four to eight points has been reported to be clinically meaningful.32 The very small absolute differences between study drugs and placebo (as well as the 95% confidence intervals) argue against inadequate sample size as the underlying reason for this negative trial. Even if the differences had been statistically significant, the small effect size would not be considered clinically relevant to support an indiscriminate clinical use of these medications.32 However, based on a post hoc analysis, modafinil and methylphenidate may have a marginal but clinically significant effect on fatigue in patients with excessive daytime sleepiness.
Methylphenidate had never been tested in a randomized clinical trial for MS-related fatigue; however, amantadine and modafinil were tested in numerous randomized trials for this clinical condition.21,22 The results of these trials were mixed: some reported amantadine and modafinil were effective for MS-related fatigue, while some did not report a benefit.12–20
Although those trials included different populations and used various designs and outcome measures, we speculate that the magnitude of the placebo effect and adequacy of masking treatment allocation might have contributed greatly to the disparate findings. The large placebo effect has been reported in several clinical trials of fatigue treatment in MS12,15,18, and other medical conditions.37 The variation in the effects of placebo on an outcome is partly explained by how a trial is conducted.38 In a parallel-group or a two-period, two-sequence crossover design, comparing an active medication against a placebo, participants can be unmasked more easily by the off-target effects of the active drug (i.e., feeling more jittery). This unmasking can have a large effect on the outcome measure. However, in a multiple crossover design, such as our study, because several active medications can have various off-target effects, unmasking is less likely. Our study design, by improving participant masking, provided results that are likely more reliable.
Based on previous conflicting trial results, we planned additional safeguards to further explore potentially negative results using several validated MS fatigue scales. For example, our primary outcome, the MFIS, has several dimensions and a 28-day look-back period while our secondary outcomes, the NeuroQoL fatigue item bank is a more recently developed questionnaire and part of the Quality of Life in Neurological Disorders tool with a 7-day look-back period. The consistency in lack of medication effect on either outcome measure in our trial provides added confidence in the results.
Fatigue (as defined as a subjective lack of physical or mental energy perceived by the individual with usual activities) is distinct from excessive daytime sleepiness. Sleepiness, defined as difficulty to stay awake and alert during the day, is less common and less severe than fatigue in patients with MS. A systematic review reported a moderate association between the ESS score and various fatigue rating scales in MS.39 There was a weak association between the MFIS total score and ESS score at the time of screening in our study. The effect of medications on fatigue may also be modified by the presence of daytime sleepiness. In our post hoc analysis, modafinil and methylphenidate improved fatigue impact more than placebo in patients with excessive daytime sleepiness. These improvements were marginally clinically significant (more than four points on MFIS total score). This observation is in line with another clinical trial of modafinil, which reported an improvement in the physical subscale of the MFIS score, only in patients with excessive daytime sleepiness.18 These results suggest that excessive daytime sleepiness or its underlying pathophysiology may contribute to fatigue impact in a subset of patients with MS. In those patients, wake-promoting agents, such as modafinil and methylphenidate, may have a marginal, but clinically significant effect in improving fatigue. These results were obtained from post hoc analyses and should be interpreted with caution.
Although these drugs did not improve fatigue more than placebo, in a post hoc exploratory analysis, a higher proportion of participants said they would choose modafinil (44%) or methylphenidate (43%) as their long-term fatigue treatment compared to placebo (32%). While this may be related to the benefits of these medications on MS symptoms other than fatigue, we also cannot exclude that the MFIS and NeuroQoL may not completely capture some dimensions of fatigue.
The majority of participants tolerated the maximum dose of all study medications. However, the total number of adverse events, moderate-to-severe adverse events, adverse events categorized as nervous system or psychiatric disorders, and the proportion of participants who reported any adverse events were higher with amantadine, modafinil, and methylphenidate compared to placebo. The higher frequency and the profile of adverse events associated with these three drugs over a short time period, as well as the risks associated with polypharmacy, should be taken into consideration when these medications are prescribed for symptom management in MS.
Our study has several major strengths. Our eligibility criteria were broad, and thus the results are generalizable. The participants were of diverse racial and ethnic background and with a wide range of MS-related disability. The multiple crossover design of the study helped preserve participant blinding to study medications (although not formally assessed), and by using each participant as his or her own control, increased the study power and promoted the recruitment. The study outcomes were all validated patient-reported metrics that are directly relevant to participants. Finally, the mixed-effect analysis method was robust against missing data, and the sensitivity analyses did not show any carry-over effect (i.e., the washout periods were adequate in length).
This study has several limitations. It was conducted at only two specialty MS clinics, and therefore, the results may not be applicable to all patients with MS. The total duration of participation in the study was relatively long due to the crossover design. If there were large-scale fluctuations in the fatigue levels over time, they might have biased the results toward the null (i.e., no medication effect). However, while diurnal and day-to-day fluctuation of fatigue are well-known phenomena in MS40, large-scale and long-term fluctuations in fatigue levels are not common41, and contrary to popular belief, fatigue severity does not fluctuate between seasons in patients with MS.42 To be closer to the pragmatic end of the pragmatic-explanatory continuum43, we did not assess treatment adherence and simplified the study procedures by minimizing the in-person visits. The results of the study could be different if the adherence was monitored, and the outcomes were measured in the clinic. MS exacerbations may change fatigue severity.6 Still, only four patients reported relapses during the study. Additionally, when we analyzed the study as a 6-week-long, parallel-group trial by only using the first treatment period outcomes, the results were not different from the overall findings. Each treatment period was short and used specific doses of medications. We cannot rule out that the results might have been different with long-term use and higher doses of study drugs. However, doses of modafinil and amantadine used in prior trials (200 to 400mg/day for modafinil and 200mg/day for amantadine) are in line with the doses we selected, and higher doses result in an increased incidence of adverse events and lower tolerability. Finally, amphetamine-like stimulants other than methylphenidate may have different effects on MS-related fatigue.
In conclusion, amantadine, modafinil, and methylphenidate were not superior to placebo in improving MS fatigue assessed by validated outcome measures and resulted in more frequent adverse events. The widespread use of these medications for MS fatigue in clinical practice is probably mostly related to a placebo effect reported by the patients. Based on our results, physicians should reduce the use of these medications for the treatment of MS-related fatigue. Our post hoc analysis suggested that, when compared with placebo, modafinil and methylphenidate might result in small improvements in fatigue in a subset of patients with excessive daytime sleepiness. Further research is needed to confirm this result and to elucidate the pathophysiology of fatigue in MS and its contributing factors, improve outcome measures, and develop effective interventions.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed articles published until June 1, 2020, without restricting our search by language. We used a combination of keywords and subject headings for the trial medications: “amantadine” OR “modafinil” OR “methylphenidate” AND “multiple sclerosis” AND “fatigue,” combined with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE (sensitivity and precision-maximising version, 2008 revision). We filtered the results for articles reporting the results of randomized clinical trials. We also reviewed the abstract from the European Committee on Treatment in Multiple Sclerosis and the American Academy of Neurology Meetings between 2006 and 2018. We did not find randomized controlled trials of methylphenidate that reported on the medication effect on MS fatigue. We found eight randomized trials of amantadine and six randomized trials of modafinil with fatigue severity as one of the study outcomes. Most, but not all of these trials were placebo-controlled. Both parallel-group and crossover designs were utilized in these trials. The duration of treatment ranged from four to 12 weeks. Various patient-reported fatigue measures were used, including, but not limited to, Modified Fatigue Impact Scale (MFIS), Fatigue Severity Scale (FSS), and visual analog scale (VAS). Three randomized, double-blind trials, which reported superiority of amantadine (200mg/day) over placebo in reducing MS-related fatigue, used visual analog scale, daily diary rating, and MS-specific fatigue scales as their outcome measures. However, one of these trials reported no effect of amantadine on the fatigue severity scale (FSS), one of the better validated and commonly used fatigue scales in MS. Another clinical trial reported a benefit of amantadine 200mg/day compared to the placebo on the MFIS score. However, it was not double-blinded, had only 15 participants in each treatment group, and there was a significant imbalance in baseline MFIS scores between the two groups. A parallel-group, randomized, double-blind, placebo-controlled study reported a statistically significant effect of amantadine 200mg/day (compared to placebo) on the FSS score but had a total sample size of 42 participants.
Multiple randomized, placebo-controlled, patient-blind, or double-blind trials with sample sizes ranging between 21 and 121 participants tested the efficacy of modafinil 200 to 400 mg/day in reducing the MFIS or FSS scores. All reported that modafinil was not superior to placebo in improving MS-related fatigue. A 72-participant, placebo-controlled trial, which reported improvement of MFIS and FSS scores with 200mg/day of modafinil (but not with 400mg/day), was a single-blind study. Another randomized, double-blind trial of modafinil 200mg/day versus placebo with 21 participants reported an improvement of FSS in the modafinil group.
Added value of this study
To our knowledge, this is the largest randomized, placebo-controlled clinical trial of fatigue medications in MS. We used the rigors of randomization, blinding, intention-to-treat analysis, and a statistical model that could accommodate missing data. Our study design, by improving subject masking, provided results that are likely more reliable. This trial was a pragmatic trial with broad eligibility criteria to improve the generalizability of the results. We also assessed the possibility of heterogeneity of treatment effects, based on several baseline characteristics. We found that improvement in fatigue severity with amantadine, modafinil, and methylphenidate was similar to placebo.
Implications of all available evidence
Our results support the notion that most of the benefits that have been reported in the clinical use of medications for MS fatigue are attributable to the placebo effect. However, there is a possibility that in a subset of patients with MS, wake-promoting agents may provide more improvement in fatigue compared to placebo. Short-term and long-term adverse events associated with these medications, along with their minimal (or no superiority) to placebo, should be considered before recommending and prescribing these medications.
Acknowledgments
Research reported in this publication was funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (MS-1511-33689). The statements presented in this article are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
We are thankful to the patients who participated in this clinical trial. We are grateful to the members of the study advisory committee and for the oversight of the data and safety monitoring boar. We thank CTSI at UCSF for its technical support.
Footnotes
Declaration of interests
BN has received funding from the National MS Society (NMSS), PCORI, and Genentech and personal fees from Jazz Pharmaceutical. CM has received funding from PCORI. EW has received personal fee from Jazz Pharmaceutical, Emerald, and DBV. KK has received funding from PCORI, NMSS and Biogen. EM has received research support from Teva, grants from Sun Pharma, Sanofi Genzyme, Biogen, and personal fees from UpToDate. NR, BM, CC, JC, MM, AR, CA, SA, CJ declare no competing interests.
Data sharing
The TRIUMPHANT-MS study protocol and statistical analysis plan will be available on request to the principal investigator (Bardia Nourbakhsh; bnourba1@jhmi.edu). Data requests should be submitted to BN for consideration. Access to available fully anonymized data may be granted 12 months after publication, after review by BN and Emmanuelle Waubant. Requesters will be asked to complete an application form detailing specific requirements, rationale, and proposed use. A data-sharing agreement will need to be signed.
Requested data will be made available, along with data dictionary on a secure server.
References
- 1.Krupp L Fatigue is intrinsic to multiple sclerosis (MS) and is the most commonly reported symptom of the disease. Mult Scler 2006; 12: 367–8. [DOI] [PubMed] [Google Scholar]
- 2.Lerdal A, Celius EG, Krupp L, Dahl AA. A prospective study of patterns of fatigue in multiple sclerosis. Eur J Neurol 2007; 14: 1338–43. [DOI] [PubMed] [Google Scholar]
- 3.Hadjimichael O, Vollmer T, Oleen-Burkey M, North American Research Committee on Multiple Sclerosis. Fatigue characteristics in multiple sclerosis: the North American Research Committee on Multiple Sclerosis (NARCOMS) survey. Health Qual Life Outcomes 2008; 6: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zifko UA. Management of Fatigue in Patients with Multiple Sclerosis. Drugs 2004; 64: 1295–304. [DOI] [PubMed] [Google Scholar]
- 5.Penner I-K, Paul F. Fatigue as a symptom or comorbidity of neurological diseases. Nat Rev Neurol 2017; 13: 662–75. [DOI] [PubMed] [Google Scholar]
- 6.Mäurer M, Comi G, Freedman MS, et al. Multiple sclerosis relapses are associated with increased fatigue and reduced health-related quality of life – A post hoc analysis of the TEMSO and TOWER studies. Multiple Sclerosis and Related Disorders 2016; 7: 33–40. [DOI] [PubMed] [Google Scholar]
- 7.Heine M, van de Port I, Rietberg MB, van Wegen EEH, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev 2015;: CD009956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalah MA, Ayache SS. Cognitive behavioral therapies and multiple sclerosis fatigue: A review of literature. J Clin Neurosci 2018; 52: 1–4. [DOI] [PubMed] [Google Scholar]
- 9.Toosy A, Ciccarelli O, Thompson A. Symptomatic treatment and management of multiple sclerosis. Handb Clin Neurol 2014; 122: 513–62. [DOI] [PubMed] [Google Scholar]
- 10.MacAllister WS, Krupp LB. Multiple Sclerosis–Related Fatigue. Physical Medicine and Rehabilitation Clinics of North America 2005; 16: 483–502. [DOI] [PubMed] [Google Scholar]
- 11.Krupp LB, Serafin DJ, Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother 2010; 10: 1437–47. [DOI] [PubMed] [Google Scholar]
- 12.Hader W, Duquette P, Auty A, et al. A randomized controlled trial of amantadine in fatigue associated with multiple sclerosis. canadian journal of neurological sciences / journal canadien des sciences neurologiques 1987; 14: 273–8. [DOI] [PubMed] [Google Scholar]
- 13.Cohen RA, Fisher M. Amantadine treatment of fatigue associated with multiple sclerosis. Arch Neurol 1989; 46: 676–80. [DOI] [PubMed] [Google Scholar]
- 14.Ledinek AH, Sajko MC, Rot U. Evaluating the effects of amantadin, modafinil and acetyl-L-carnitine on fatigue in multiple sclerosis--result of a pilot randomized, blind study. Clin Neurol Neurosurg 2013; 115 Suppl 1: S86–89. [DOI] [PubMed] [Google Scholar]
- 15.Krupp LB, Coyle PK, Doscher C, et al. Fatigue therapy in multiple sclerosis: results of a double-blind, randomized, parallel trial of amantadine, pemoline, and placebo. Neurology 1995; 45: 1956–61. [DOI] [PubMed] [Google Scholar]
- 16.Brioschi A, Gramigna S, Werth E, et al. Effect of modafinil on subjective fatigue in multiple sclerosis and stroke patients. Eur Neurol 2009; 62: 243–9. [DOI] [PubMed] [Google Scholar]
- 17.Rammohan KW, Rosenberg JH, Lynn DJ, Blumenfeld AM, Pollak CP, Nagaraja HN. Efficacy and safety of modafinil (Provigil) for the treatment of fatigue in multiple sclerosis: a two centre phase 2 study. J Neurol Neurosurg Psychiatr 2002; 72: 179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stankoff B, Waubant E, Confavreux C, et al. Modafinil for fatigue in MS: a randomized placebo-controlled double-blind study. Neurology 2005; 64: 1139–43. [DOI] [PubMed] [Google Scholar]
- 19.Möller F, Poettgen J, Broemel F, Neuhaus A, Daumer M, Heesen C. HAGIL (Hamburg Vigil Study): a randomized placebo-controlled double-blind study with modafinil for treatment of fatigue in patients with multiple sclerosis. Mult Scler 2011; 17: 1002–9. [DOI] [PubMed] [Google Scholar]
- 20.Lange R, Volkmer M, Heesen C, Liepert J. Modafinil effects in multiple sclerosis patients with fatigue. J Neurol 2009; 256: 645–50. [DOI] [PubMed] [Google Scholar]
- 21.Pucci E, Branãs P, D’Amico R, Giuliani G, Solari A, Taus C. Amantadine for fatigue in multiple sclerosis. Cochrane Database Syst Rev 2007; : CD002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng P, Hou L, Wang X, et al. Efficacy of modafinil on fatigue and excessive daytime sleepiness associated with neurological disorders: a systematic review and meta-analysis. PLoS ONE 2013; 8: e81802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinshenker BG, Penman M, Bass B, Ebers GC, Rice GP. A double-blind, randomized, crossover trial of pemoline in fatigue associated with multiple sclerosis. Neurology 1992; 42: 1468–71. [DOI] [PubMed] [Google Scholar]
- 24.Geisler MW, Sliwinski M, Coyle PK, Masur DM, Doscher C, Krupp LB. The effects of amantadine and pemoline on cognitive functioning in multiple sclerosis. Arch Neurol 1996; 53: 185–8. [DOI] [PubMed] [Google Scholar]
- 25.Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol 2010; 28: 3673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerr CW, Drake J, Milch RA, et al. Effects of methylphenidate on fatigue and depression: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage 2012; 43: 68–77. [DOI] [PubMed] [Google Scholar]
- 27.Horadam VW, Sharp JG, Smilack JD, et al. Pharmacokinetics of amantadine hydrochloride in subjects with normal and impaired renal function. Ann Intern Med 1981; 94: 454–8. [DOI] [PubMed] [Google Scholar]
- 28.Darwish M, Kirby M, Hellriegel ET, Robertson P. Armodafinil and modafinil have substantially different pharmacokinetic profiles despite having the same terminal half-lives: analysis of data from three randomized, single-dose, pharmacokinetic studies. Clin Drug Investig 2009; 29: 613–23. [DOI] [PubMed] [Google Scholar]
- 29.Kimko HC, Cross JT, Abernethy DR. Pharmacokinetics and clinical effectiveness of methylphenidate. Clin Pharmacokinet 1999; 37: 457–70. [DOI] [PubMed] [Google Scholar]
- 30.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Annals of Neurology 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Téllez N, Río J, Tintoré M, Nos C, Galán I, Montalban X. Does the Modified Fatigue Impact Scale offer a more comprehensive assessment of fatigue in MS? Mult Scler 2005; 11: 198–202. [DOI] [PubMed] [Google Scholar]
- 32.Rooney S, McFadyen DA, Wood DL, Moffat DF, Paul PL. Minimally important difference of the fatigue severity scale and modified fatigue impact scale in people with multiple sclerosis. Multiple Sclerosis and Related Disorders 2019; 35: 158–63. [DOI] [PubMed] [Google Scholar]
- 33.Cella D, Lai J-S, Nowinski CJ, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology 2012; 78: 1860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14: 540–5. [DOI] [PubMed] [Google Scholar]
- 35.Kos D, Duportail M, D’hooghe M, Nagels G, Kerckhofs E. Multidisciplinary fatigue management programme in multiple sclerosis: a randomized clinical trial. Mult Scler 2007; 13: 996–1003. [DOI] [PubMed] [Google Scholar]
- 36.Nourbakhsh B, Revirajan N, Waubant E. Association Between Glutamate Blockade and Fatigue in Patients With Multiple Sclerosis. JAMA Neurol 2015; 72: 1374–5. [DOI] [PubMed] [Google Scholar]
- 37.Hoenemeyer TW, Kaptchuk TJ, Mehta TS, Fontaine KR. Open-Label Placebo Treatment for Cancer-Related Fatigue: A Randomized-Controlled Clinical Trial. Sci Rep 2018; 8: 2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev 2010; : CD003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popp RFJ, Fierlbeck AK, Knüttel H, et al. Daytime sleepiness versus fatigue in patients with multiple sclerosis: A systematic review on the Epworth sleepiness scale as an assessment tool. Sleep Med Rev 2017; 32: 95–108. [DOI] [PubMed] [Google Scholar]
- 40.Powell DJH, Liossi C, Schlotz W, Moss-Morris R. Tracking daily fatigue fluctuations in multiple sclerosis: ecological momentary assessment provides unique insights. J Behav Med 2017; 40: 772–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koch M, Uyttenboogaart M, Harten A van, Heerings M, Keyser JD. Fatigue, depression and progression in multiple sclerosis: Multiple Sclerosis Journal 2008; published online July 1. DOI: 10.1177/1352458508088937. [DOI] [PubMed] [Google Scholar]
- 42.Bakalidou D, Giannopoulos S, Stamboulis E, Voumvourakis K. Effect of seasonal fluctuation of ambient temperature on fatigue in multiple sclerosis patients living in Attica, Greece. Journal of Clinical Neuroscience 2014; 21: 1188–91. [DOI] [PubMed] [Google Scholar]
- 43.Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. CMAJ 2009; 180: E47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


