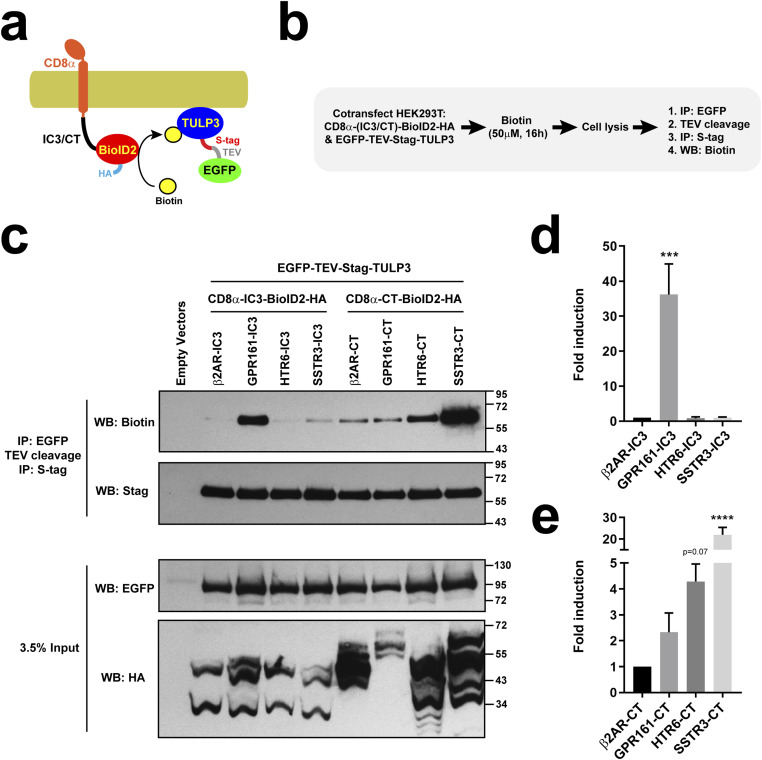
Figure 9. HTR6 and SSTR3 CTs associate with ciliary trafficking adapter TULP3.
(A, B) Schematic and protocol of BioID2 proximity labeling assay. HEK293T cells were cotransfected with plasmids encoding EGFP-TEV-Stag-TULP3 and a fusion protein containing the extracellular and transmembrane regions of CD8α (aa 1–206), the C-terminal tail (CT) or third intracellular loop (IC3) of a G protein-coupled receptor, the BioID2 biotin ligase, and an HA epitope. In presence of biotin (50 μM, 16 h), BioID2 biotinylates surrounding proteins in a proximity-dependent manner. After cell lysis, TULP3 was affinity purified by two sequential immunoprecipitations (IP) and its biotinylation assessed by Western blot (WB). (C) SDS–PAGE and WB analysis of immunoprecipitated S-tagged TULP3 (top two panels) and of the cleared cell lysates (bottom two). In the IPs, NeutrAvidin-HRP was used to detect TULP3 biotinylation (top) and anti-Stag antibody to detect its total levels. In the lysates, anti-EGFP and anti-HA tag antibodies were used to detect EGFP-TEV-Stag-TULP3 and the CD8 fusions, respectively. Molecular weight markers are indicated on the right (kD). (C, D, E) Biotinylated TULP3 signal, relative to total TULP3 signal in IPs, was quantitated from n = 5 independent experiments like the one in (C). Biotinylation by IC3 constructs (D) and by CT constructs (E) was normalized relative to β2AR-IC3 and β2AR-CT, respectively. Data are mean ± SEM and were analyzed by one-way ANOVA followed by Dunnett’s multiple comparison tests. Significance shown as P < 0.0001 (****).
Source data are available for this figure.