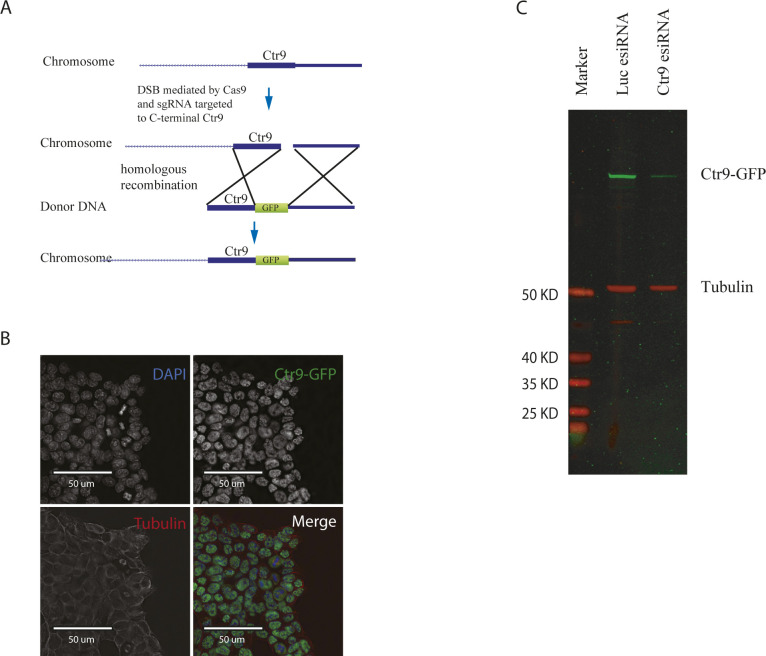
Figure S1. Generation of Ctr9-GFP knock-in mES cells.
(A) Schematic presentation of the targeting strategy. GFP knock-in to the C terminus of Ctr9 by CRISPR/Cas9–mediated homologous recombination. Cas9 nuclease and an short guide RNA targeted to the C-terminus of Ctr9 were co-transfected to make a double-stranded break in the mouse ESCs genome. The double-strand break is repaired by homologous recombination with a donor template containing GFP flanked by homologous arms for Ctr9, where GFP is designed to be integrated. (B) Cellular localization of the Ctr9-GFP fusion protein. The Ctr9-GFP fusion protein was stained in green by using an anti-GFP antibody. Tubulin was stained in red by an anti-tubulin antibody. The cell nucleus was stained in blue by DAPI. Note the nuclear localization of the Ctr9-GFP fusion protein. (C) esiRNA knockdown of Ctr9. Mouse embryonic stem cells expressing the Ctr9-GFP fusion protein were transfected with esiRNAs targeting Ctr9. The non-targeting esiRNA (Luc esiRNA) served as control. Anti-GFP antibody was used to visualize the fusion protein at around 160 kD (green channel), and anti-tubulin antibody was used to detect tubulin (50 kD, red channel) as the loading control. Specific depletion of Ctr9-GFP fusion protein is seen in the Ctr9 knockdown sample.