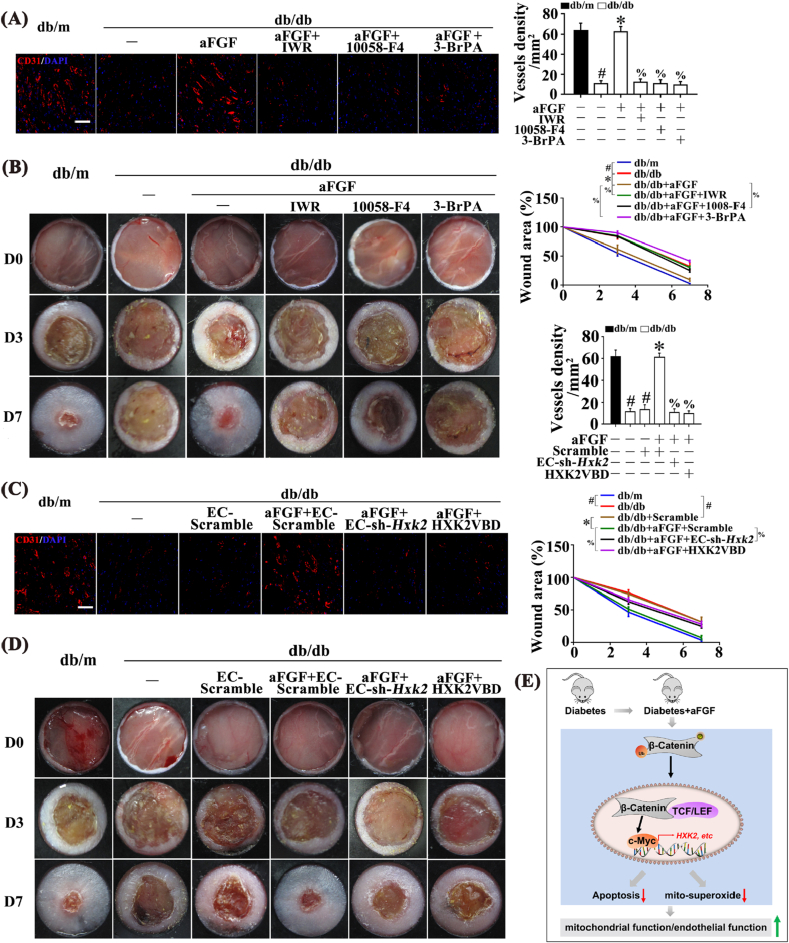
Fig. 7.
Inhibition of Wnt/β-catenin-c-Myc-HXK2 pathway and/or interference with mitochondrial location of HXK2 abrogated aFGF-prompted wound healing in T2DM. (A) Confocal immunofluorescence staining with CD31 of wounded skin tissue sections at 7 days post wounding, scale bars = 30 μm, and (B) Images of skin wounds from db/m mice, db/db mice and db/db mice receiving aFGF (100 ng/mL) treatment. For signaling pathway analysis, ICG-001 (10 μM) or 10058-F4 (50 μM) or BrPA (50 μM) was injected intradermally into the wound edges in the mice after aFGF treatment. (C) Confocal immunofluorescence staining with CD31 of wounded skin tissue sections at 7 days post wounding, scale bars = 30 μm, and (D) images of skin wounds from db/m mice, db/db mice and db/db mice receiving aFGF (100 ng/mL) treatment. Ad-sh-HXK2 was injected intradermally into the wound edges in the mice the day before wounding, HXK2VBD peptide (100 μM) was injected intradermally into the wound edges in the mice after aFGF treatment. (E) Schematic showing that aFGF alleviated diabetes-induced endothelial impairment by downregulating mtROS via Wnt/β-catenin pathway. All values displayed are means ± SEM of 6 independent experiments. #p < 0.05 vs. db/m mice; *p < 0.05 vs. db/db mice; % p < 0.05 vs. aFGF treated db/db mice.