Abstract
Well-differentiated neuroendocrine tumors (NETs) arising in the gastrointestinal (GI) tract and pancreas are relatively rare; however, the annual incidence has been increasing. Carcinoid syndrome (CS) is a constellation of symptoms that occur when a GI NET metastasizes to the liver and releases high levels of vasoactive substances into the systemic circulation. CS occurs in 19% of NETs patients at diagnosis and is associated with shorter survival. Carcinoid heart disease (CHD) occurs in over 50% of patients with CS and is associated with poor long-term prognosis. NET-induced valvular fibrosis is a significant cause of mortality and morbidity in these patients. Somatostatin analogs relieve CS symptoms, but they have never been shown to reverse CHD progression or improve overall survival. Surgical therapy for right-sided valve disease is associated with improved symptoms and quality of life and possibly improved survival, despite relatively high morbidity and mortality associated with cardiac intervention. A 65-year-old woman with a metastatic pancreatic NET had typical signs and symptoms of CS. She presented in congestive heart failure and was found to have severe tricuspid regurgitation with characteristic features of CHD on transthoracic echocardiogram (TTE). Following octreotide monotherapy, serial TTEs demonstrated regression of tricuspid valve involvement. The patient improved clinically and remained asymptomatic on subsequent visits. This is the first case of CHD regression with medical therapy supported by serial TTEs. Developing a deeper understanding of cases like this will help us unlock new intervention targets and strategies for treatments in the future.
Keywords: Neuroendocrine tumor, Carcinoid heart disease, Somatostatin analog
Introduction
Well-differentiated neuroendocrine tumors (NETs) arising in the gastrointestinal (GI) tract and pancreas are relatively rare. The annual incidence in the United States is approximately 3.56 per 100,000 and has been rising over time [1]. “Carcinoid syndrome” (CS) is a constellation of symptoms that occur when a GI NET metastasizes to the liver and releases high levels of vasoactive substances into the systemic circulation. CS occurs in 19% of NETs patients at the time of diagnosis and is associated with shorter survival [2]. CS manifestations include flushing, diarrhea, bronchospasm, and development of carcinoid heart disease (CHD) [3].
CHD eventually occurs in over 50% of patients with CS and may be the initial presentation of NETs in as many as 20% of patients [4]. Heart failure (HF) secondary to right-sided valvular fibrosis has emerged as one of the most serious extraintestinal manifestations of NETs, occurring in 20–70% of patients with metastatic disease and resulting in as much as 50% of NETs mortality [5]. Cardiac involvement is associated with a poor long-term prognosis: estimated 3-year survival rates of 31% are half that of patients without CHD [6, 7, 8]. The main predictor of prognosis is the presence of severe structural and functional abnormalities of the tricuspid valve (TV) [8].
CHD occurs when large amounts of vasoactive substances such as serotonin, tachykinins, and prostaglandins reach the right heart [7]. Generally, the left heart is spared because lungs metabolize vasoactive substances; rare exceptions are associated with patent foramen ovale (PFO), primary bronchopulmonary carcinoid disease, and severe disease burden [4]. After the initial endocardial injury, plaque is deposited on endocardial surfaces of right-sided heart valves, papillary muscles, chordae tendineae, and along the walls of the atria and ventricles. The plaque is composed of fibroblasts, smooth muscle cells, and extracellular matrix [6, 9]. Fibrosis may be induced by serotonin, evidenced by high levels of serotonin metabolites found in urine of CHD patients and the presence of serotonin receptors on their cardiac valves [10, 11]. Characteristic changes on echocardiography include thickening of valve leaflets and cusps that become retracted and eventually immobile; this results in a combination of right-sided valvular regurgitation and stenosis [7].
There is currently no medical therapy effective in ameliorating carcinoid-induced fibrosis [12]. Loop and thiazide diuretics are used to control symptoms. They must be carefully titrated to optimize fluid status while avoiding volume depletion, which exacerbates fatigue and lowers cardiac output [6]. Somatostatin analogs octreotide and lanreotide inhibit tumoral serotonin secretion and lower 5-hydroxyindoleacetic acid (5-HIAA) levels, thus relieving CS symptoms. However, these medications neither reverse CHD progression nor improve overall survival [12]. As a consequence, timely diagnosis and early surgical correction with valve replacement in appropriately selected patients is the only effective treatment. Surgical therapy for right-sided valve disease is associated with improvement of symptoms and quality of life, and it may contribute to improved survival [12]. Unfortunately, cardiac surgical intervention in this population is associated with relatively high morbidity and mortality [9, 12].
We report the first case of CHD regression on serial transthoracic echocardiograms (TTE) following octreotide monotherapy.
Case Report
In September 2017, a 65-year-old female with multiple comorbidities was diagnosed with metastatic pancreatic NET and CHD. Her past medical history was significant for hypertension, dyslipidemia, chronic obstructive pulmonary disease, hypothyroidism, migraines, gastroesophageal reflux disease, essential tremor, depression, anxiety, osteoporosis, and chronic back pain due to lumbar stenosis. She was an active smoker with a 50-pack-year smoking history. She previously suffered from alcohol dependence but had not consumed a drink in several years. She also had a history of pain medication over-use in the setting of her chronic back pain. She is a retired secretary with 3 healthy adult children and no significant cardiac or oncologic family history. The patient's home medications at the time of starting octreotide can be found in Table 1. Her clinical course is described below.
Table 1.
Home medications at the time of starting octreotide in December 2017
| Home medications |
|---|
| 1. Alendronate |
| 2. Levothyroxine |
| 3. Nifedipine |
| 4. Perindopril |
| 5. Olanzapine |
| 6. Pantoprazole |
| 7. Acetylsalicylic acid |
| 8. Fluticasone/Salmeterol |
| 9. Tiotropium |
| 10. Perindopril |
| 11. Pramipexole |
| 12. Codeine |
From 2008 to 2017, the patient had several admissions to hospital for nausea, emesis, diarrhea, and abdominal pain and cramping. No definitive cause was determined for most of these episodes. She was diagnosed with acute pancreatitis likely due to alcohol ingestion in 2010 and a colonic biopsy demonstrated collagenous colitis following a colonoscopy in 2012. The patient was also empirically diagnosed with Addison's disease due to persistent severe hypokalemia in the setting of her chronic GI symptoms. This diagnosis was later rescinded and fludrocortisone was discontinued.
In July 2015, the patient was admitted to hospital for 1 month for a right-sided parapneumonic effusion that failed conservative management. She underwent a thoracoscopic decortication and pleurectomy and did well post-operatively. During this admission, a TTE was done to rule out infective endocarditis. The August 2015 TTE demonstrated mildly elevated estimated right ventricular systolic pressure but was otherwise unremarkable (Table 2).
Table 2.
Serial transthoracic echocardiogram reports
| Serial TTE reports | ||||
|---|---|---|---|---|
| Study date | 11th Aug 15 | 28th Jun 17 | 17th Oct 17 | 25th Nov 19 |
| Study location | RAH | outpatient | MAHI | MAHI |
| LV size | normal | normal | normal | normal |
| LV systolic function | normal | normal | normal | normal |
| RV size | normal | borderline enlarged | severely dilated | normal |
| RV systolic function | normal | normal | normal | normal |
| RA size | normal | moderate-to-severely enlarged | severely dilated | normal |
| LA size | normal | normal | normal | normal |
| TV morphology | normal | “poor coaptation of leaflets with relatively immobile leaflet” | “valvular appearance consistent with CHD” | grossly normal |
| TR | trace | severe | severe | mild-to-moderate |
| PV morphology | not well seen | – | grossly normal | grossly normal |
| PR | – | moderate-to-severe | severe | moderate |
| RVSP estimate | 43 mm Hg | 42 mm Hg | at least 45 mm Hg | 36 mm Hg |
| MV morphology | normal | normal | thickened MV leaflets | normal |
| MR | none | none | mild | trace |
| AV morphology | normal | sclerosis | sclerosis | sclerosis |
| AR | none | moderate-to-severe | moderate | mild |
RAH, Royal Alexandra Hospital; MAHI, Mazankowski Alberta Heart Institute; TTE, transthoracic echocardiogram.
The patient remained out of hospital until the following year when she was admitted for recurring abdominal pain and diarrhea in July 2016. This time her stool initially tested positive for Clostridium difficile toxin B gene. Despite a prolonged course of appropriate anti-microbial therapy and all subsequent stool samples testing negative for C. difficile toxin, her diarrhea did not improve.
The patient's diarrhea and abdominal pain and cramping persisted along with significant weight loss over the course of the next year, which triggered further testing. An outpatient abdominal ultrasound done in May 2017 showed multiple hyperechoic foci within the liver that were deemed most likely to be hemangiomas.
A follow-up outpatient abdominal computed tomography (CT) scan was arranged, but before it could be done, the patient presented to her local emergency department with worsening dyspnea, bilateral lower extremity edema, and orthopnea in June 2017. She was admitted to hospital with predominantly right-sided new-onset congestive HF. She responded well to intravenous furosemide and was discharged home 3 days later on oral furosemide. At discharge the patient's HF was deemed to be primarily due to “lung pathology” against a background of poorly controlled hypertension as per her discharge summary report.
An outpatient TTE in June 2017 noted significant changes compared to her previously described 2015 TTE study (Table 2). The etiology of this new severe right-sided heart disease was thought to be due to pulmonary hypertension resulting from underlying chronic obstructive pulmonary disease and left ventricular diastolic dysfunction.
Over the course of the next few months, the patient's diarrhea, abdominal pain, and weight loss persisted. A follow-up outpatient abdominal and pelvic CT scan in August 2017 demonstrated multiple enhancing lesions throughout the liver, a suspected pulmonary nodule, and multiple sclerotic foci throughout the axial skeleton. The report stated that “metastatic carcinoid tumor” was the “diagnosis of exclusion”. A mass in the body of the pancreas was also described. The report also commented on “dilation of the right heart with splaying of the TV and clear visualization of the TV leaflets” and “altered bolus timing and distention of the right heart suggestive of associated right heart dysfunction (endocardial fibroelastosis) secondary to presumed CS.”
One month later, in September 2017, the patient underwent an endoscopic ultrasound (EUS) and two pancreatic body masses were identified. The larger mass was biopsied and the pathology report for both specimens confirmed a well-differentiated pancreatic NET with a Ki-67 index less than 2% (grade I). The second smaller mass was not biopsied; however, the EUS report stated that this smaller mass was suggestive of a “classic NET” upon inspection with EUS imaging alone. Her chromogranin A (CgA) level at the time was elevated at 376 ng/mL (normal ≤110 ng/mL). A 24-h urinary 5-HIAA level was not reported.
Unfortunately, following the EUS-guided biopsy the patient was admitted to the intensive care unit for hemorrhagic shock due to severe intra-abdominal bleeding. She also developed peritonitis from an infected hematoma and was treated with a prolonged course of broad-spectrum antibiotics over the course of her 2-month admission.
A whole-body 18F-DOPA (6-[18F]-L-fluoro-L-3,4-dihydroxyphenylalanine) positron emission tomography (PET) scan done during this admission demonstrated an intensely 18F-DOPA-avid NET associated with the terminal ileum and adjacent mesentery with hepatic and multifocal skeletal metastatic disease. Interestingly, the skeletal metastatic disease in the manubrium and bilateral scapulae had been present on studies dating back to at least December 2011. These skeletal lesions appeared to be largely stable, consistent with indolent disease. There was also focal 18F-DOPA uptake in the pancreas demonstrating the pancreatic NETs previously identified on EUS. An octreotide scan confirmed that the majority of the 18F-DOPA-avid disease was also octreotide-avid. A favorable response to somatostatin analog therapy was thus predicted.
The patient underwent a repeat TTE in October 2017 at the Mazankowski Alberta Heart Institute (MAHI) 2 weeks prior to discharge. This TTE supported new significant right-sided findings of the outpatient TTE done in June 2017 (Table 2; Fig. 1, 2). The patient was euvolemic at the time of discharge with normal renal function and was not prescribed any diuretics (Table 1). Outpatient follow-up was arranged with oncology for initiation of treatment.
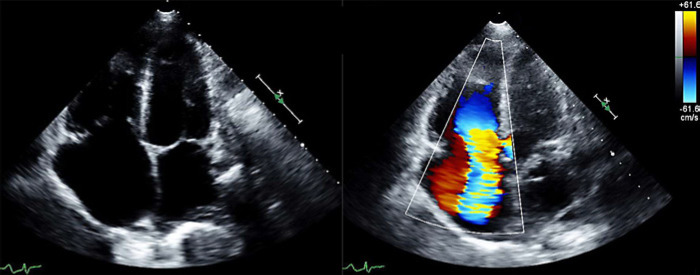
Fig. 1.
Oct 17, 2017: Apical 4 chamber view of TV in systole: 2D and color Doppler. Valvular appearance consistent with CHD and severe TR.
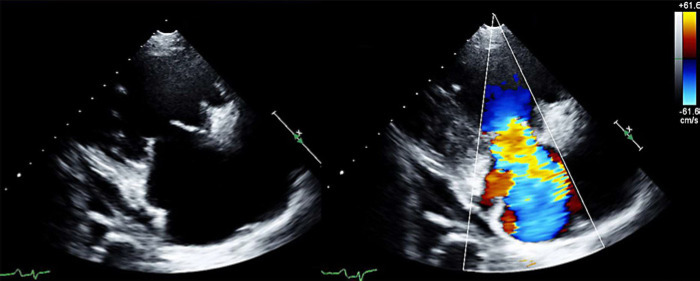
Fig. 2.
Oct 17, 2017: Parasternal long axis view of TV in systole: 2D and color Doppler. Valvular appearance consistent with CHD and severe TR.
In December 2017, the patient was started on octreotide injections (Sandostatin LAR Depot) 30 mg i.m. every 28 days. She had initially been considered for peptide receptor radionuclide therapy, but due to her dramatic improvement on octreotide monotherapy and increased risk of nephrotoxicity in the setting of developing renal disease, peptide receptor radionuclide therapy was withheld.
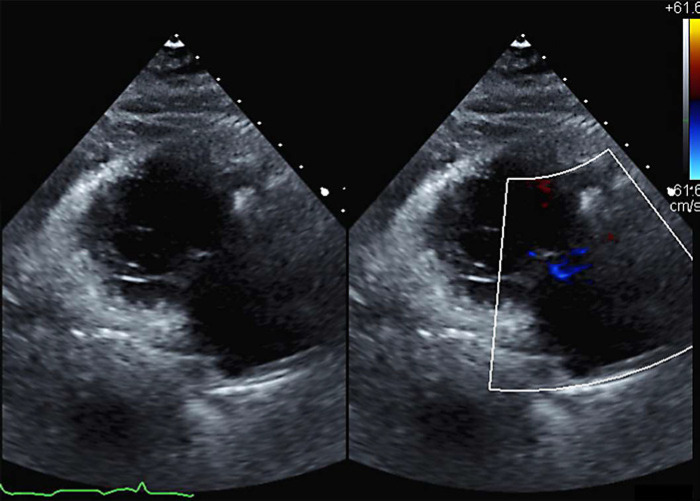
In November 2019, after nearly 2 years of treatment with octreotide monotherapy, a repeat TTE was done at the MAHI to re-assess her CHD. This study demonstrated significant improvement compared to the October 17, 2017 TTE (Table 2). The patient now had normal right ventricle (RV) size and systolic function (from “severely dilated RV with preserved function”), normal right atrium (RA) size (from “severely dilated RA”), grossly normal TV morphology with mild to moderate tricuspid regurgitation (TR) (from “wide open severe TR” and “valvular appearance consistent with CHD”), grossly normal pulmonary valve (PV) with moderate pulmonary regurgitation (PR) (from “severe PR”), aortic sclerosis with mild aortic regurgitation (AR) (from “moderate AR”), and trace MR (from “mild MR”). Of note, the anterior TV leaflet was reported to have “decent mobility, and certainly not looking fixed and immobile” as previously reported (Table 2; Fig. 3).
Fig. 3.
Nov 25, 2019: Parasternal long axis view of TV in systole: 2D and color Doppler. Grossly normal morphology with mild-to-moderate TR.
At her most recent oncology clinic follow-up visit in January 2020, the patient reported that she was doing well clinically and had “no carcinoid symptoms” as per the clinic note. She specifically denied any pedal edema, orthopnea, or paroxysmal nocturnal dyspnea, and she was not taking any diuretics. She had no complaints of nausea, emesis, diarrhea, or abdominal pain. An octreotide scan in August 2019 and a CT scan in May 2020 showed stable findings. In December 2019, a 24-h urinary 5-HIAA was within normal limits (32 μmol/day, normal range 0–40 μmol/day) and her CgA level was 236 ng/mL, significantly decreased from 376 ng/mL in September 2017 (normal ≤110 ng/mL).
At present, the patient is 68 years old and was recently diagnosed with mild dementia by Geriatric Medicine. Otherwise, she remains clinically well on monthly intramuscular octreotide injections with no evidence of disease progression.
Discussion/Conclusion
Regression of NET-induced leaflet fibrosis and resultant valve dysfunction has never before been reported in the literature [13]. This is first case of regression of CHD with medical therapy in a patient with metastatic pancreatic NETs supported by serial TTEs.
Fixed, immobile, and retracted leaflets are typical of CHD and can contribute to significant regurgitation and stenosis [7]. This typical morphology was clearly depicted on TTE at the time of NET diagnosis (Fig. 1) and it was also reflected on the August 2017 CT scan, which described “splaying of the TV with clear visualization of the TV leaflets”.
One confounding factor when it comes to TR severity is that TR is load-dependent, so its severity is affected by the patient's volume status. If our patient was more euvolemic at the time of her follow-up TTE, this may have contributed to improvements in TR that were observed. Normalization of RA and RV sizes reduce TV annular dilatation as well. Nevertheless, improved volume status and decreased right-sided chamber enlargement with reduced annular stretch would not account for the normalization of TV morphology and significantly improved leaflet mobility that was observed on the 2019 TTE (Fig. 3). These echocardiographic findings demonstrate that either significant reversal of TV fibrosis is possible or that the patient was intervened upon early enough to reverse an inflammatory process that may be a precursor to frank fibrosis.
This patient likely exhibited left-sided valve involvement as well. Aortic valve (AV) “sclerosis” and “thickening” of the mitral valve (MV) leaflets with moderate AR and mild MR at the time of diagnosis may have developed due to deposition of carcinoid plaques in the left heart. We do not have a 5-HIAA level from the time of diagnosis to offer biochemical insight into the circulating levels of serotonin, but extensive metastases indicate that her disease burden at the time of diagnosis was high. It is possible that the patient's pulmonary enzymes were unable to adequately shield the left heart from significantly elevated levels of serotonin and other vasoactive mediators. There was no evidence of a PFO on color Doppler; however, a PFO cannot be ruled out. Following octreotide treatment, moderate AR and mild MR regressed to mild AR and trace MR. Left-sided valvular lesions improved along with the right-sided ones.
In addition to left heart involvement, carcinoid plaque may also involve the endocardial lining of the heart chambers in patients with advanced disease [4]. The CT scan from August 2017 commented on “altered bolus timing and distention of the right heart suggestive of associated right heart dysfunction (endocardial fibroelastosis) secondary to presumed CS.” Endocardial fibroelastosis is characterized by diffuse thickening of the endocardium secondary to proliferation of fibrous and elastic tissue [14] and in this case likely reflects carcinoid plaque deposition on endocardial surfaces of the RV, which can be seen in the setting of severe disease [4]. Subsequent CT scans did not comment on this, so it is unclear if endocardial RV carcinoid plaques regressed following octreotide therapy. TTE images did not demonstrate any dense echoes along the RV endocardial surface, which would suggest RV plaque deposition.
NET-induced valvular fibrosis is a significant cause of mortality and morbidity in patients with metastatic NET disease [5]. Surgical therapy for right-sided valve disease is associated with improved symptoms and quality of life and possibly improved survival, despite the relatively high morbidity and mortality associated with cardiac intervention [12]. Our newfound understanding that carcinoid plaque deposition and NET-induced intracardiac fibrosis is a potentially reversible process following octreotide therapy is an important realization that offers many avenues for further study. An underlying genetic basis for this novel response to octreotide therapy must be explored so that it may be eventually exploited as our proficiency with genomics evolves. Artificial intelligence-powered techniques are leading us into the new age of precision medicine by helping us decode heterogeneous pathophysiological factors and processes that contribute to disease [15]. Developing a deeper understanding of cases like this one and how and why they differ from the norm will help us unlock new intervention targets and strategies for treatments in the future.
Statement of Ethics
The patient was unable to provide informed consent due to dementia. Written informed consent was obtained from the patient's husband for publication of this case report and accompanying images. The patient's husband is her legal representative/agent. She has granted him power of attorney and he makes all decisions on her behalf regarding her care.
Conflict of Interest Statement
Michael Sawyer has honoraria with Novartis < USD 5,000 and travel support to conferences < USD 10 000. Ermin Nath and Jonathan Choy declare that they have no conflict of interest.
Funding Sources
None.
Author Contributions
All 3 authors meet the 4 criteria for authorship as outlined by the ICMJE.
Dr. Ermin Nath: made substantial contributions toward the composition of the manuscript as well as the acquisition, analysis, and interpretation of the data. Participated in drafting the case report and revising it critically for important intellectual content. Dr. Nath has given final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Dr. Michael Sawyer: made substantial contributions toward the composition of the manuscript as well as the acquisition, analysis, and interpretation of the data. Participated in drafting the case report and revising it critically for important intellectual content. Dr. Sawyer has given final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Dr. Jonathan Choy: made substantial contributions toward composition of the manuscript as well as the acquisition, analysis, and interpretation of the data. Participated in drafting the case report and revising it critically for important intellectual content. Dr. Choy has given final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017 Oct;3((10)):1335–42. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halperin DM, Shen C, Dasari A, Xu Y, Chu Y, Zhou S, et al. The frequency of carcinoid syndrome at neuroendocrine tumor diagnosis: A large population-based study using SEER-Medicare data. Lancet Oncol. 2017 Apr;18((4)):525–34. doi: 10.1016/S1470-2045(17)30110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med. 1999 Mar;340((11)):858–68. doi: 10.1056/NEJM199903183401107. [DOI] [PubMed] [Google Scholar]
- 4.Pellikka PA, Tajik AJ, Khandheria BK, Seward JB, Callahan JA, Pitot HC, et al. Carcinoid Heart Disease. Clinical and Echocardiographic Spectrum in 74 Patients. Circulation. 1993 Apr;87((4)):1188–96. doi: 10.1161/01.cir.87.4.1188. [DOI] [PubMed] [Google Scholar]
- 5.Zuetenhorst J, Taal BG. Metastatic carcinoid tumors (a clinical review) Oncologist. 2005 Feb;10((2)):123–31. doi: 10.1634/theoncologist.10-2-123. [DOI] [PubMed] [Google Scholar]
- 6.Moller JE, Pellikka PA, Bernheim AM, Schaff HV, Rubin J, Connolly HM. Prognosis of carcinoid heart disease: analysis of 200 cases over 2 decades. Circulation. 2005 Nov;112((21)):3320–7. doi: 10.1161/CIRCULATIONAHA.105.553750. [DOI] [PubMed] [Google Scholar]
- 7.Fox DJ, Khattar RS. Carcinoid heart disease: presentation, diagnosis, and management. Heart. 2004 Oct;90((10)):1224–8. doi: 10.1136/hrt.2004.040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westberg G, Wängberg B, Ahlman H, Bergh CH, Beckman-Suurküla M, Caidahl K. Prediction of prognosis by echocardiography in patients with midgut carcinoid syndrome. Br J Surg. 2001 Jun;88((6)):865–72. doi: 10.1046/j.0007-1323.2001.01798.x. [DOI] [PubMed] [Google Scholar]
- 9.Grozinsky-Glasberg S, Grossman AB, Gross DJ. Carcinoid heart disease: from pathophysiology to treatment –‘something in the way it moves’. Neuroendocrinology. 2015 Jul;101((4)):263–73. doi: 10.1159/000381930. [DOI] [PubMed] [Google Scholar]
- 10.Gustafsson BI, Hauso O, Drozdov I, Kidd M, Modlin IM. Carcinoid heart disease. Int J Cardiol. 2008 Oct;129((3)):318–24. doi: 10.1016/j.ijcard.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Zuetenhorst JM, Bonfrer JM, Korse CM, Bakker R, van Tinteren H, Taal B. Carcinoid heart disease: the role of urinary 5-hydroxyindoleacetic acid excretion and plasma levels of atrial natriuretic peptide, transforming growth factor-beta and fibroblast growth factor. Cancer. 2003 Apr;97((7)):1609–15. doi: 10.1002/cncr.11226. [DOI] [PubMed] [Google Scholar]
- 12.Modlin IM, Latich I, Kidd M, Zikusoka M, Eick G. Therapeutic options for gastrointestinal carcinoids. Clin Gastroenterol Hepatol. 2006 May;4((5)):526–47. doi: 10.1016/j.cgh.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Ram P, Penalver JL, Lo KBU, Rangaswami J, Pressman GS. Carcinoid Heart Disease: Review of Current Knowledge. Tex Heart Inst J. 2019 Feb;46((1)):21–7. doi: 10.14503/THIJ-17-6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schork NJ. Chapter 11: Artificial Intelligence and Personalized Medicine. In: Von Hoff DD, Han H, editors. Precision Medicine in Cancer Therapy. Cancer Therapy and Research. Cham, Switzerland: Springer; 2019. pp. p. 265–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denfield SW, Gajarski RJ, Towbin JA. Cardiomyopathies. In: Garson A Jr, Bricker JT, Fisher DJ, Neish SR, editors. Science and Practice of Pediatric Cardiology. 2nd ed. Baltimore: Williams and Wilkins; 1998. p. p. 1851. [Google Scholar]