Abstract
Background:
To examine the association between malignant peritoneal cytology and survival in women with early-stage endometrioid endometrial cancer.
Methods:
This is a retrospective cohort study using the Surveillance, Epidemiology, and End Results Program from 2010 to 2016. Women with stage I endometrioid endometrial cancer who had peritoneal cytology testing at hysterectomy were examined (N = 24,800). Characteristics and survival related to malignant peritoneal cytology were assessed. The propensity score inverse probability of treatment weighting was used to balance the measured covariates.
Findings:
Malignant peritoneal cytology was reported in 1081 (4.4%) women. In multivariable analysis, stage IB disease and moderately/poorly differentiated tumours were associated with an increased likelihood of malignant peritoneal cytology (both P < 0.05). In a weighted model, malignant peritoneal cytology was associated with decreased cause-specific survival (5-year rates, 92.1% versus 96.8%, hazard ratio [HR] 1.98, 95% confidence interval [CI] 1.56–2.52) and overall survival (89.4% versus 93.1%, HR 1.41, 95% CI 1.16–1.72). In sensitivity analyses, malignant peritoneal cytology was associated with decreased overall survival in the high–intermediate-risk group (5-year rates, 77.8% versus 83.6%, HR 1.57, 95% CI 1.20–2.06) and decreased cause-specific survival in the low-risk group (95.4% versus 98.0%, HR 1.64, 95% CI 1.01–2.68). In the high–intermediate-risk group with malignant peritoneal cytology, postoperative chemotherapy was associated with improved overall survival compared to whole pelvic radiotherapy (5-year rates, 82.7% versus 64.6%, HR 0.36, 95% CI 0.14–0.96). This association was not observed in negative cytology cases (81.5% versus 79.7%, HR 0.78, 95% CI 0.53–1.14).
Interpretation:
Malignant peritoneal cytology may be associated with decreased survival in stage I endometrioid endometrial cancer.
Keywords: Endometrioid endometrial cancer, Peritoneal cytology, Malignant peritoneal cytology, Survival
1. Introduction
Globally, endometrial cancer remains the most common gynaecologic malignancy in developed countries such as Europe and the United States of America (USA) [1]. Endometrial cancer commonly presents as an early-stage endometrioid tumour [2,3], and a hysterectomy-based surgery is the standard treatment for this disease [4]. Endometrial cancer is staged surgically based on tumour factors identified in the surgical specimen, and tailored postoperative therapy is based on these factors to reduce disease relapse [4,5]. Thus, identifying additional prognostic factors can potentially further optimise the postoperative management of endometrial cancer.
One such prognostic factor may be malignant peritoneal cytology, which was an element of the Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) cancer staging system before the 2009 revision [6]. The removal of malignant peritoneal cytology from the cancer staging system was presumably due to the lack of a prognostic impact in some publications [7,8]. However, multiple studies have also demonstrated worse oncologic outcomes in the setting of malignant peritoneal cytology [9–20], and the current National Comprehensive Cancer Network (NCCN) guidelines continue to recommend the evaluation of peritoneal cytology at the time of hysterectomy [4]. As the above-mentioned studies are limited either by small sample sizes or non-stratification according to histology type or cancer stage, further studies are warranted to assess the prognostic impact of malignant peritoneal cytology in endometrial cancer patients.
Mounting evidence supports that endometrioid and non-endometrioid tumours have distinct molecular and clinical characteristics [2,21]. Early-stage endometrioid endometrial cancer represents the most common subgroup and carries a favourable prognosis, but the survival impact of malignant peritoneal cytology specific to this subset of patients has not been thoroughly examined [22]. The objective of this study was to examine the association between malignant peritoneal cytology and survival in women with early-stage endometrioid endometrial cancer.
2. Materials and methods
2.1. Data source
This is a retrospective observational study examining the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program [23]. The SEER program is the largest population-based tumour registry in the USA and has been in operation for more than four decades. The program covers ~35% of the US population in the latest version, and their data are publicly available and deidentified. Patient identification, data entry and rigorous quality control for the program are managed by registered trained personnel [24]. University of Southern California Institutional Review Board exempted this study because of the use of deidentified, publicly available data.
2.2. Study eligibility
Women with stage I (T1/N0-x/M0-x) endometrioid endometrial cancer who had peritoneal cytology performed at the time of hysterectomy were examined from 2010 to 2016. The starting point of 2010 was chosen because FIGO revised the cancer staging schema in 2009 and the SEER program has information for peritoneal cytology testing during the study period. Patients with stages II–IV disease, an unknown tumour stage, with non-endometrioid tumours, a secondary tumour diagnosis or who did not undergo hysterectomy were excluded from the analysis. Peritoneal cytology results with suspicious, but not confirmed malignant cytology were also excluded.
2.3. Clinical information
Information abstracted from the database included patient demographics, tumour characteristics, treatment type and survival outcomes. Patient demographics included age, year of diagnosis, race/ethnicity, registry area, insurance status and marital status. Tumour characteristics included tumour differentiation, T-stage, tumour size and peritoneal cytology results. Treatment types included hysterectomy type, performance of pelvic and para-aortic lymph node dissection, number of sampled nodes and postoperative therapy modalities. Survival outcomes included follow-up duration after the endometrial cancer diagnosis, vital status and cause of death. Survival status in the program is externally linked to the National Death Index for validation [25].
2.4. Study definition
For the exposure group allocation, Collaborative Stage Site-Specific Factor 2 was used to interpret the peritoneal cytology result as malignant peritoneal cytology (code, 010) or negative peritoneal cytology (code, 000) [26,27]. For the outcome measures, cause-specific survival (CSS) was defined as the interval between the endometrial cancer diagnosis and death due to endometrial cancer. Overall survival (OS) was defined as the interval between the endometrial cancer diagnosis and death from any cause. Women who had no survival event at the last follow-up were censored.
For the subcohorts, the high–intermediate-risk group was defined as the presence of ≥2 of the following three risk factors: age ≥60 years, high-grade tumour and myometrial invasion ≥50% per the PORTEC definition [28]. This group also included poorly differentiated tumour with myometrial invasion ≥50%. The low-risk group was defined as stage IA low-grade endometrioid tumours modified by the NCCN guidelines [4].
Histology type was based on the International classification of diseases for oncology, 3rd edition (ICD-O-3)/World Health Organisation histological classification as described previously [29]. The American Joint Committee on Cancer (AJCC) staging classification schema was used for defining T-stage. Low-grade tumour was defined as well- and moderately-differentiated tumours and high-grade tumour was defined as poorly differentiated tumour [30,31]. Lymphadenectomy performance was verified with the SEER coding for “Regional Nodes” that was introduced in 1988 and has not changed since. Postoperative therapy was grouped based on the use of radiotherapy and chemotherapy types.
2.5. Statistical considerations
The primary step of the analysis was to identify the independent characteristics associated with malignant peritoneal cytology. A binary logistic regression model was fitted, and all the pre-/intra-operative factors with a P value <0.05 in the univariable analysis were entered into the final multivariable model. Multicollinearity was assessed with variance inflation factor and the Hosmer–Lemeshow test was used to assess the goodness-of-fit in the final model. A P value >0.05 was interpreted as a good-fit model [32]. Effect size was expressed with adjusted odds ratio and 95% confidence interval (CI).
The second step of the analysis was to assess the survival outcomes related to malignant peritoneal cytology. The propensity score inverse probability of treatment weighting (PS-IPTW) method was used to balance the potential confounding of measured covariates between the two groups [33]. The PS was computed by fitting a multivariable binary logistic regression model for the malignant peritoneal cytology status [34]. All measured covariates were entered into the model. The PS-IPTW approach assigned women with malignant peritoneal cytology a weight of 1/PS and those who had negative peritoneal cytology were assigned a weight of 1/(1-PS). Stabilised weights were used, and the threshold technique was used at the first and 99th percentile of the weight distribution [33].
The size effect in the weighted model was assessed between the two groups, and a standardised difference (SD) of >0.20 was interpreted as the presence of size effect for clinical imbalance between the groups [35]. The Kaplan–Meier method was used to construct survival curves, and Cox proportional hazard regression models were fitted to estimate hazard ratio (HR) with 95% CI for malignant peritoneal cytology. The proportional hazard assumption was tested and satisfied without interaction over time.
Various sensitivity analyses were undertaken to assess the robustness of the study results. First, the significance of malignant peritoneal cytology on survival outcomes was assessed in the high–intermediate-risk and the low-risk group. Second, survival outcomes were assessed per postoperative therapy types (whole pelvic radiotherapy [WPRT] versus chemotherapy) in the high-–intermediate-risk group, stratified by the peritoneal cytology results. Third, association of malignant peritoneal cytology and survival outcome was assessed in low-grade and high-grade tumours. Fourth, doubly robust adjustment was performed for the covariates exhibiting size effect between the two groups in the weighted model (SD > 0.2). Last, a generalized boosted model, a class of machine learning method, was fitted to determine the weight as an alternative analytic approach.
All statistical analyses were based on two-sided hypotheses and a P value <0.05 was considered to be statistically significant. Statistical Package for Social Sciences (version 25.0, Armonk, NY, USA) and R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria) were used for analyses. The STROBE guidelines were consulted to outline this observational cohort study [36].
A systematic review and meta-analysis were undertaken to assess the survival impact of malignant peritoneal cytology in early-stage endometrial cancer. The detailed methodology is described in Methods S1.
3. Results
Among 53,636 women in the initial search, 24,800 women met the inclusion criteria (Fig. 1). Malignant peritoneal cytology was reported in 1081 (4.4%) women. In the univariable analysis (Tables 1 and S1), age, race/ethnicity, registry area, tumour differentiation, cancer stage, performance of pelvic and para-aortic lymphadenectomy as well as tumour size were significantly associated with malignant peritoneal cytology (all P < 0.05). In the multivariable analysis (Table 1), stage IB disease (7.0% versus 3.8%) and moderately/poorly differentiated tumours (5.0–6.8% versus 3.6%) remained independent tumour factors for an increased likelihood of malignant peritoneal cytology (both P < 0.05). Women with malignant peritoneal cytology were more likely to receive any type of postoperative therapy compared to those with negative cytology (38.2% versus 18.8%, P < 0.001).
Fig. 1. CONSORT study schema.
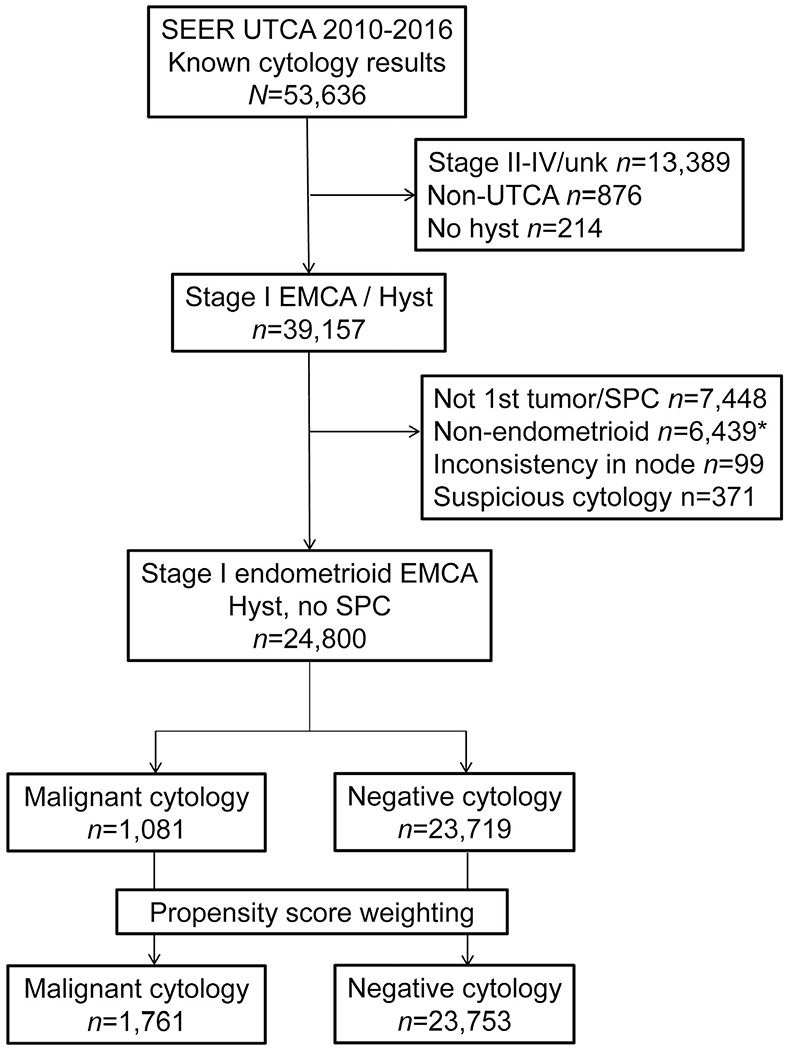
*Included sarcoma cases. SEER, Surveillance, Epidemiology, and End Result Program; UTCA, uterine cancer; unk, unknown; EMCA, endometrial cancer; SPC, secondary primary cancer; cytology; peritoneal cytology; hyst, hysterectomy.
Table 1.
Patient demographics per peritoneal cytology status (N = 24,800).
| Characteristics |
Negative |
Malignant |
P-value | aOR (95% CI) | P-value† |
|---|---|---|---|---|---|
| No. | n = 23,719 | n = 1081 | |||
| Age (yr) | 0.022 | <0.001* | |||
| <40 | 800 (3.4%) | 29 (2.7%) | 1.36 (0.90–2.07) | 0.146 | |
| 40–49 | 2176 (9.2%) | 88 (8.1%) | 1.48 (1.12–1.97) | 0.007 | |
| 50–59 | 7570 (31.9%) | 382 (35.3%) | 1.63 (1.33–2.00) | <0.001 | |
| 60–69 | 8512 (35.9%) | 388 (35.9%) | 1.34 (1.10–1.64) | 0.004 | |
| 70–79 | 3584 (15.1%) | 135 (12.5%) | 1 | ||
| ≥80 | 1077 (4.5%) | 59 (5.5%) | 1.39 (1.01–1.90) | 0.044 | |
| Year | 0.445 | ||||
| 2010 | 3265 (13.8%) | 130 (12.0%) | |||
| 2011 | 3231 (13.6%) | 137 (12.7%) | |||
| 2012 | 3346 (14.1%) | 144 (13.3%) | |||
| 2013 | 3248 (13.7%) | 158 (14.6%) | |||
| 2014 | 3525 (14.9%) | 175 (16.2%) | |||
| 2015 | 3631 (15.3%) | 173 (16.0%) | |||
| 2016 | 3473 (14.6%) | 164 (15.2%) | |||
| Race/ethnicity | <0.001 | <0.001* | |||
| White | 16,846 (71.0%) | 801 (74.1%) | 1 | ||
| Black | 1529 (6.4%) | 35 (3.2%) | 0.48 (0.34–0.67) | <0.001 | |
| Hispanic | 2674 (11.3%) | 111 (10.3%) | 0.80 (0.65–0.99) | 0.042 | |
| Asian | 2250 (9.5%) | 119 (11.0%) | 0.98 (0.80–1.21) | 0.841 | |
| Others | 203 (0.9%) | 2 (0.2%) | 0.20 (0.05–0.80) | 0.023 | |
| Unknown | 217 (0.9%) | 13 (1.2%) | 1.19 (0.67–2.10) | 0.554 | |
| Marital status | 0.312 | ||||
| Single | 4486 (18.9%) | 234 (21.6%) | |||
| Married | 12,847 (54.2%) | 561 (51.9%) | |||
| Divorced | 2453 (10.3%) | 105 (9.7%) | |||
| Separated | 209 (0.9%) | 7 (0.6%) | |||
| Widowed | 2504 (10.6%) | 112 (10.4%) | |||
| Unmarried/domestic | 79 (0.3%) | 5 (0.5%) | |||
| Unknown | 1141 (4.8%) | 57 (5.3%) | |||
| Insurance | 0.792 | ||||
| Yes | 22,883 (96.5%) | 1047 (96.9%) | |||
| No | 553 (2.3%) | 22 (2.0%) | |||
| Unknown | 283 (1.2%) | 12 (1.1%) | |||
| Registry area | <0.001 | <0.001* | |||
| West | 12,681 (53.5%) | 617 (57.1%) | 1 | ||
| Central | 4521 (19.1%) | 125 (11.6%) | 0.55 (0.45–0.68) | <0.001 | |
| East | 6517 (27.5%) | 339 (31.4%) | 1.08 (0.93–1.24) | 0.326 | |
| Tumour differentiation | <0.001 | <0.001* | |||
| Well | 10,488 (44.2%) | 394 (36.4%) | 1 | ||
| Moderate | 5280 (22.3%) | 275 (25.4%) | 1.24 (1.05–1.45) | 0.010 | |
| Poor | 1826 (7.7%) | 133 (12.3%) | 1.56 (1.26–1.93) | <0.001 | |
| Unknown | 6125 (25.8%) | 279 (25.8%) | 1.14 (0.97–1.33) | 0.118 | |
| T-stage | <0.001 | <0.001* | |||
| IA | 18,870 (79.6%) | 738 (68.3%) | 1 | ||
| IB | 4246 (17.9%) | 320 (29.6%) | 1.66 (1.43–1.92) | 0.001 | |
| I NOS | 603 (2.5%) | 23 (2.1%) | 1.06 (0.69–1.62) | 0.535 | |
| Pelvic lymphadenectomy | 14 (IQR 9–20) | 14 (IQR 9–20) | <0.001 | 0.002* | |
| No | 8405 (35.4%) | 264 (24.4%) | 1 | ||
| Yes | 15,218 (64.2%) | 814 (75.3%) | 1.32 (1.13–1.55) | 0.001 | |
| Unknown | 96 (0.4%) | 3 (0.3%) | 0.60 (0.12–3.03) | 0.535 | |
| Para-aortic lymphadenectomy | 5 (IQR 3–9) | 5 (IQR 9–20) | <0.001 | 0.039* | |
| No | 16,755 (70.6%) | 659 (61.0%) | 1 | ||
| Yes | 6819 (28.7%) | 416 (38.5%) | 1.20 (1.04–1.39) | 0.012 | |
| Unknown | 145 (0.6%) | 6 (0.6%) | 1.42 (0.45–4.50) | 0.552 | |
| Tumour size (cm) | <0.001 | 0.014* | |||
| ≤2.0 | 5662 (23.9%) | 201 (18.6%) | 1 | ||
| 2.1–4.0 | 7528 (31.7%) | 398 (36.8%) | 1.27 (1.06–1.52) | 0.008 | |
| 4.1–6.0 | 3825 (16.1%) | 207 (19.1%) | 1.20 (0.98–1.47) | 0.083 | |
| 6.1–8.0 | 1179 (5.0%) | 51 (4.7%) | 0.93 (0.68–1.29) | 0.678 | |
| >8.0 | 540 (2.3%) | 33 (3.1%) | 1.29 (0.88–1.91) | 0.196 | |
| Unknown | 4985 (21.0%) | 191 (17.7%) | 0.97 (0.79–1.20) | 0.798 | |
| Hysterectomy type | 0.391 | ||||
| Simple | 22,114 (93.2%) | 1004 (92.9%) | |||
| Modified/radical | 755 (3.2%) | 35 (3.2%) | |||
| Supracervical | 213 (0.9%) | 6 (0.6%) | |||
| NOS | 637 (2.7%) | 36 (3.3%) | |||
| Postoperative therapy | <0.001 | ||||
| None | 19,263 (81.2%) | 668 (61.8%) | |||
| VBT | 2887 (12.2%) | 173 (16.0%) | |||
| WPRT | 949 (4.0%) | 79 (7.3%) | |||
| VBT/chemo | 239 (1.0%) | 64 (5.9%) | |||
| Chemo | 205 (0.9%) | 64 (5.9%) | |||
| WPRT/chemo | 140 (0.6%) | 31 (2.9%) | |||
| RT NOS | 31 (0.1%) | 1 (0.1%) | |||
| RT NOS/chemo | 5 (<0.1%) | 1 (0.1%) |
Number (% per group) or median (IQR) is shown. Percentage per row is shown in Table S2. Chi-square test for univariable analysis. A binary logistic regression model for multivariable analysis. All the preoperative and operative covariates with P <0.05 in univariable analysis were entered in the final model.
P-value for multivariable analysis.
P-value for interaction. Hosmer–Lemeshow test shows P = 0.230 indicating a good-fit model.
aOR, adjusted odds ratio; CI, confidence interval; NOS, not otherwise significant; VBT, vaginal brachytherapy; WPRT, whole pelvic radiotherapy; RT, radiotherapy; chemo, chemotherapy.
In a pre-weighted model, adjuvant therapy, cancer stage, lymphadenectomy and registry area exhibited clinical size effect (all SD > 0.2; Fig. S1). In a PS-IPTW model, the measured covariates were overall more balanced than in the pre-weighted model between the two groups (SD ≤ 0.20), and there was a small size effect noted in adjuvant therapy (SD 0.253; Fig. S1). A total of 1761 women with malignant peritoneal cytology were compared to 23,753 women with negative peritoneal cytology. The median follow-up was 3.0 years, and there were 1130 deaths including 554 deaths related to endometrial cancer.
Women in the malignant peritoneal cytology group had significantly lower 5-year CSS (92.1% versus 96.8%) and OS (89.4% versus 93.1%) rates compared to those in the negative cytology group (both P < 0.001; Fig. 2A and B). Estimated HRs for endometrial cancer death and all-cause mortality related to malignant peritoneal cytology were 2.32 (95% CI 1.81–2.94) and 1.52 (95% CI 1.25–1.85), respectively. After controlling for adjuvant therapy (Fig. 3), this association remained independent: CSS, HR 1.98 (95% CI 1.56–2.52) and OS, HR 1.41 (95% CI 1.16–1.72).
Fig. 2. The survival curve estimates based on peritoneal cytology results (whole cohort).
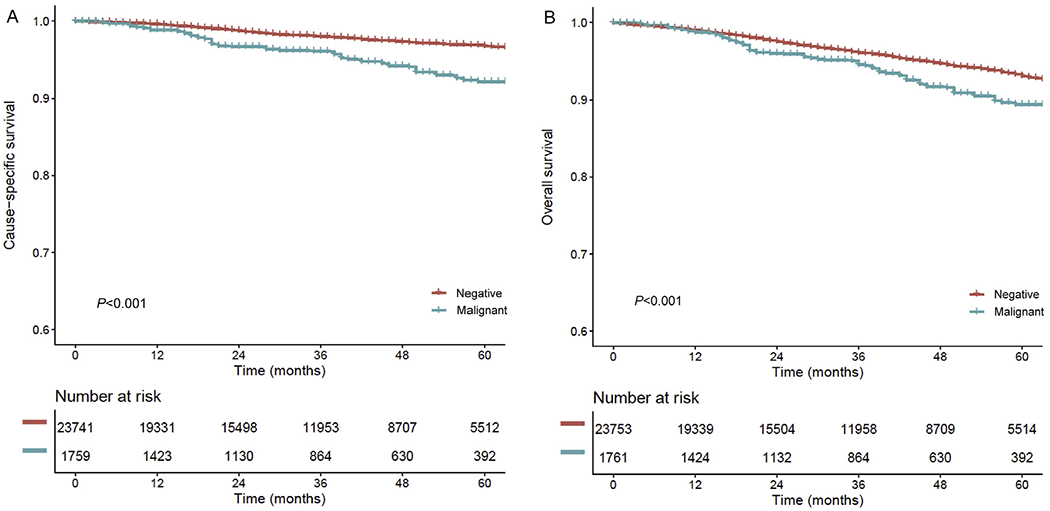
Survival curves are shown per peritoneal cytology status: (A) cause-specific survival and (B) overall survival. Cox proportional hazard regression model for P-value. Y-axis is truncated to 0.6–1.0. Negative, no malignant cells in peritoneal cytology; malignant, malignant cells in peritoneal cytology.
Fig. 3. Forest plots for outcome measures.
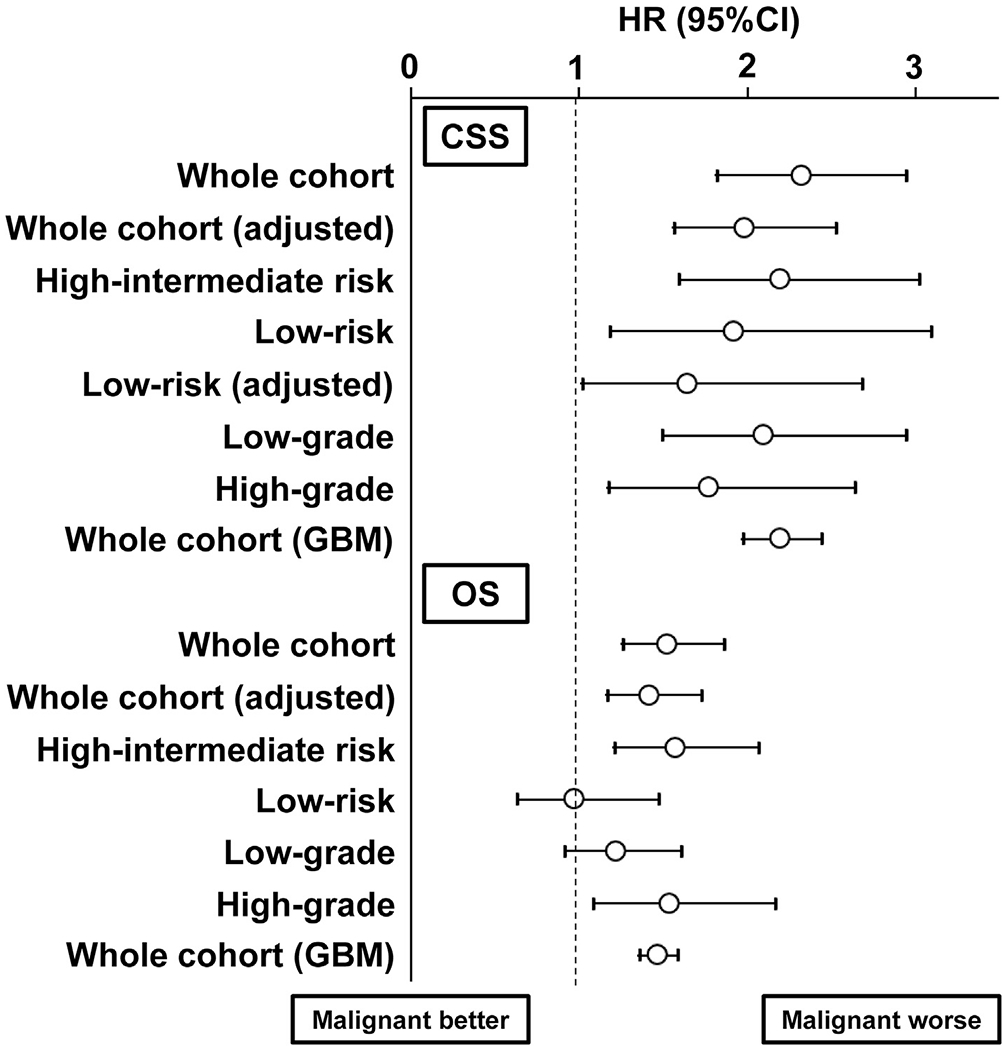
An association of peritoneal cytology (malignant versus negative) and survival outcome was assessed with Cox proportional hazard regression in the PS-IPTW models. The adjusted model indicates that the association of malignant peritoneal cytology and survival outcome was adjusted for the covariates exhibiting size effect in weighted model. Circles represent HR, and bars represent 95% CI. HR, hazard ratio; CI, confidence interval; WPRT, whole pelvic radiotherapy; GBM, generalized boosted model; CSS, cause-specific survival; OS, overall survival.
In the high–intermediate-risk group (n = 4346), 277 women (6.4%) had malignant peritoneal cytology. In the PS-IPTW model (n = 4484; Fig. S2), all measured covariates were clinically balanced (SD ≤ 0.20) and malignant peritoneal cytology was associated with decreased CSS (5-year rates, 81.6% versus 91.3%, HR 2.19, 95% CI 1.59–3.02, P < 0.001) and OS (77.8% versus 83.6%, HR 1.57, 95% CI 1.20–2.06, P = 0.001) compared to negative peritoneal cytology (Fig. 4A and B).
Fig. 4. The survival curve estimates based on peritoneal cytology results (high–intermediate-risk and low-risk groups).
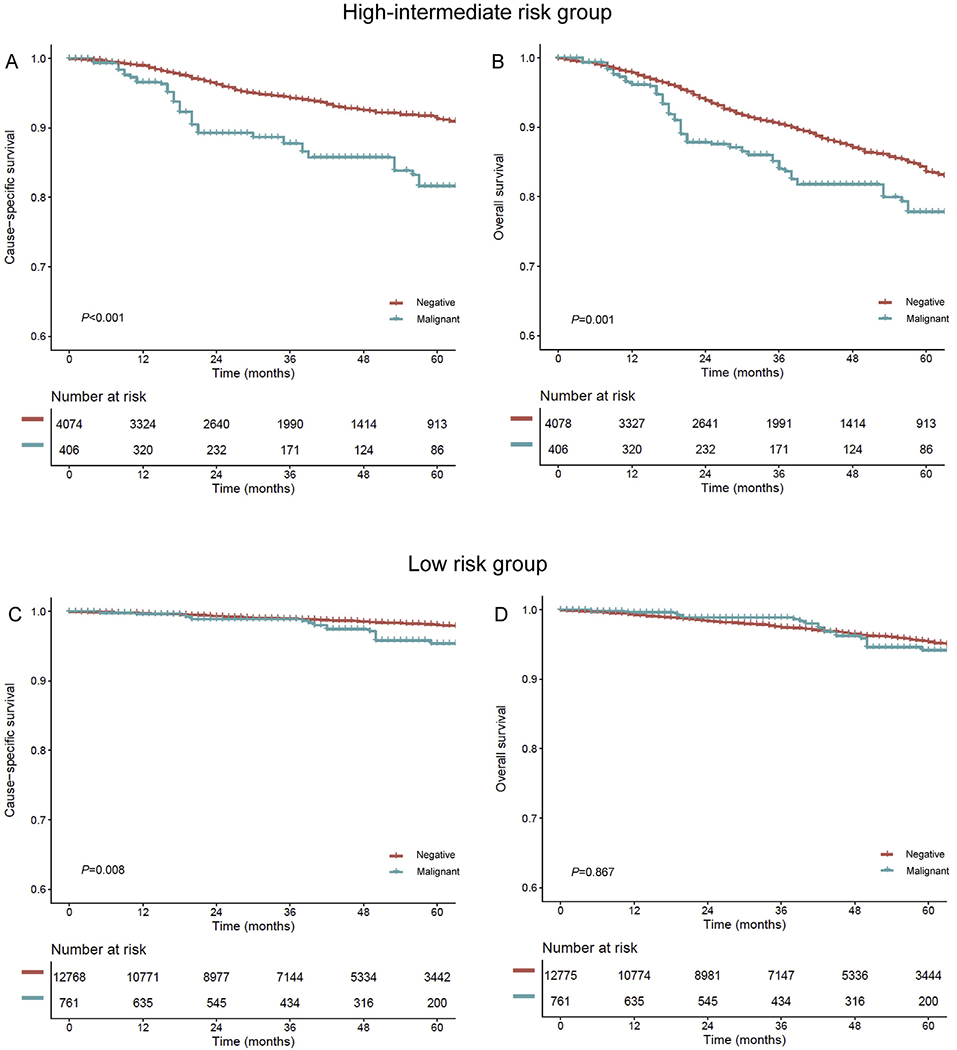
Survival curves based on peritoneal cytology status (malignant versus negative) are shown for (A) CSS and (B) OS in the high–intermediate-risk group and (C) CSS and (D) OS in the low-risk group. Y-axis is truncated to 0.6–1.0. Negative, no malignant cells in peritoneal cytology; malignant, malignant cells in peritoneal cytology; CSS, cause-specific survival; OS, overall survival.
In the low-risk group (n = 13,220), malignant peritoneal cytology was recorded in 459 women (3.5%). In a PS-IPTW model (n = 13,536; Fig. S3), adjuvant therapy showed a small size effect for clinical imbalance between the two groups (SD 0.239). Women in the malignant peritoneal cytology group had significantly decreased CSS (5-year rates, 95.4% versus 98.0%, HR 1.91, 95% CI 1.18–3.09, P = 0.008; Fig. 4C) compared to those in the negative peritoneal cytology group. After controlling for adjuvant therapy (Fig. 3), the association remained independent (HR 1.64, 95% CI 1.01–2.68, P = 0.049). OS was similar between the two groups (5-year rates, 94.2% versus 95.4%, HR 0.96, 95% CI 0.63–1.47, P = 0.867; Fig. 4D).
In an exploratory analysis, postoperative therapy modalities were compared in the high–intermediate-risk group. Among women with negative peritoneal cytology (Fig. 5A and B), chemotherapy and WPRT showed similar CSS (5-year rates, 83.6% versus 85.1%, HR 0.87, 95% CI 0.57–1.32, P = 0.508) and OS (81.5% versus 79.7%, HR 0.78, 95% CI 0.53–1.14, P = 0.204). In contrast, among women with malignant peritoneal cytology (Fig. 5C and D), postoperative chemotherapy use was associated with improved OS compared to WPRT use (5-year rates, 82.7% versus 64.6%, HR 0.36, 95% CI 0.14–0.96, P = 0.040). Albeit statistically nonsignificant, a similar trend was observed for CSS (5-year rates, 84.7% versus 75.8%, HR 0.45, 95% CI 0.15–1.36, P = 0.158).
Fig. 5. The survival curve estimates based on postoperative therapy (high–intermediate-risk cohort).
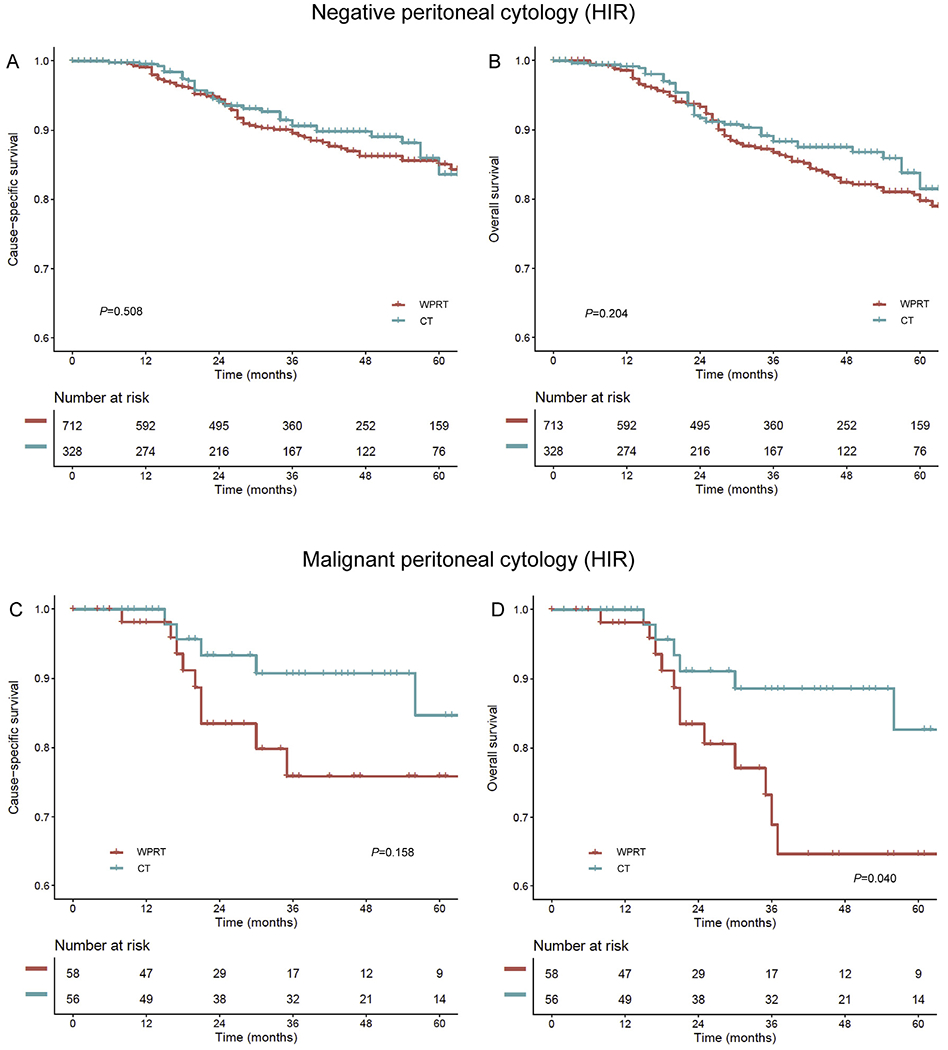
Survival curves based on postoperative therapy status (chemotherapy versus whole pelvic radiotherapy) are shown for (A) CSS and (B) OS in the negative cytology group and (C) CSS and (D) OS in the malignant cytology group. Malignant peritoneal cytology cases were examined in the unweighted model because of limited sample size and event number. All measured factors were balanced in negative cytology cases (all SD ≤ 0.2). Y-axis is truncated to 0.6–1.0. WPRT, whole pelvic radiotherapy; CT, chemotherapy; CSS, cause-specific survival; OS, overall survival.
The impact of malignant peritoneal cytology on CSS was non-differential across tumour grades (Fig. 3): low-grade tumours, 5-year rates 94.6% versus 97.5%, HR 2.09, 95% CI 1.49–2.94, P < 0.001 and high-grade tumours, 78.2% versus 88.9%, HR 1.76, 95% CI 1.17–2.64, P = 0.006. Malignant peritoneal cytology was associated with decreased OS in high-grade tumours (5-year rates, 72.9% versus 82.8%, HR 1.53, 95% CI 1.08–2.16, P = 0.016) but not in low-grade tumours (92.9% versus 94.2%, HR 1.21, 95% CI 0.91–1.60, P = 0.193).
Last, the generalized boosted model was used to examine the effect size of malignant peritoneal cytology on survival outcomes. All the covariates were well-balanced without clinical size effect between the two groups (all SD ≤ 0.2; Fig. S4). In this model, malignant peritoneal cytology was associated with increased risk of endometrial cancer death (HR 2.19, 95% CI 1.97–2.44, P < 0.001) and all-cause death (HR 1.46, 95% CI 1.35–1.58, P < 0.001; Fig. 3).
A systematic review identified 14 studies (Fig. S4 and Table S2), and a meta-analysis was carried out combining these with the present study (15 studies, n = 79,393) [7–20]. All studies were of a retrospective cohort design. Eight studies (53.3%) examined malignant peritoneal cytology in only endometrioid tumours [8,10,13,15,17,18,20], and of these, three studies (20.0%) solely examined stage I endometrioid endometrial cancer [10,13,17]. The frequency of malignant peritoneal cytology ranged widely from 1.6% to 20.6% (Fig. 6). On compiling all the study samples, the average frequency of malignant peritoneal cytology was 5.2% for stages I–II endometrial cancer, 7.4% for stages I–II endometrioid endometrial cancer and 3.6% for stage I endometrioid endometrial cancer.
Fig. 6. Frequency of malignant peritoneal cytology in early-stage endometrial cancer (systematic review).
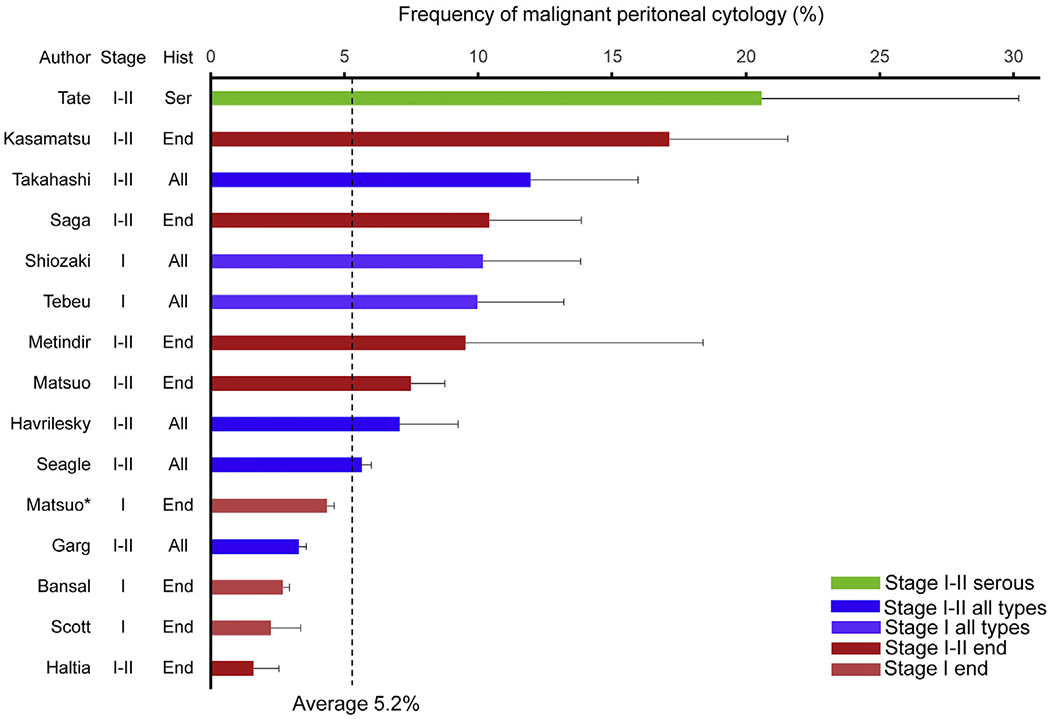
Percent frequency of malignant peritoneal cytology is shown per study, with 95% confidence interval. Average of malignant peritoneal cytology per summation of all study samples was 5.2%. Malignant peritoneal cytology rates were 20.6% for stages I–II serous, 10.1% for stage I all histology types, 7.4% for stages I–II endometrioid, 4.7% for stages I–II all histologies and 3.6% for stage I endometrioid types. *Present reported study. Hist, histology; Ser, serous; End, endometrioid; All, all histology types.
In a pooled analysis of stages I–II disease with all histology types (Fig. 7A and B), malignant peritoneal cytology was associated with decreased disease-free survival (HR 3.81, 95% CI 2.13–6.80, P < 0.001; 7 studies) and OS (HR 1.71, 95% CI 1.41–2.08, P < 0.001; 6 studies). When restricted to stage I endometrioid endometrial cancer, this association remained significant for disease-free survival (HR 2.27, 95% CI 1.12–4.58, P = 0.020) and OS (HR 1.40, 95% CI 1.15–1.71, P < 0.001).
Fig. 7. Results of meta-analysis for survival impact of malignant peritoneal cytology.
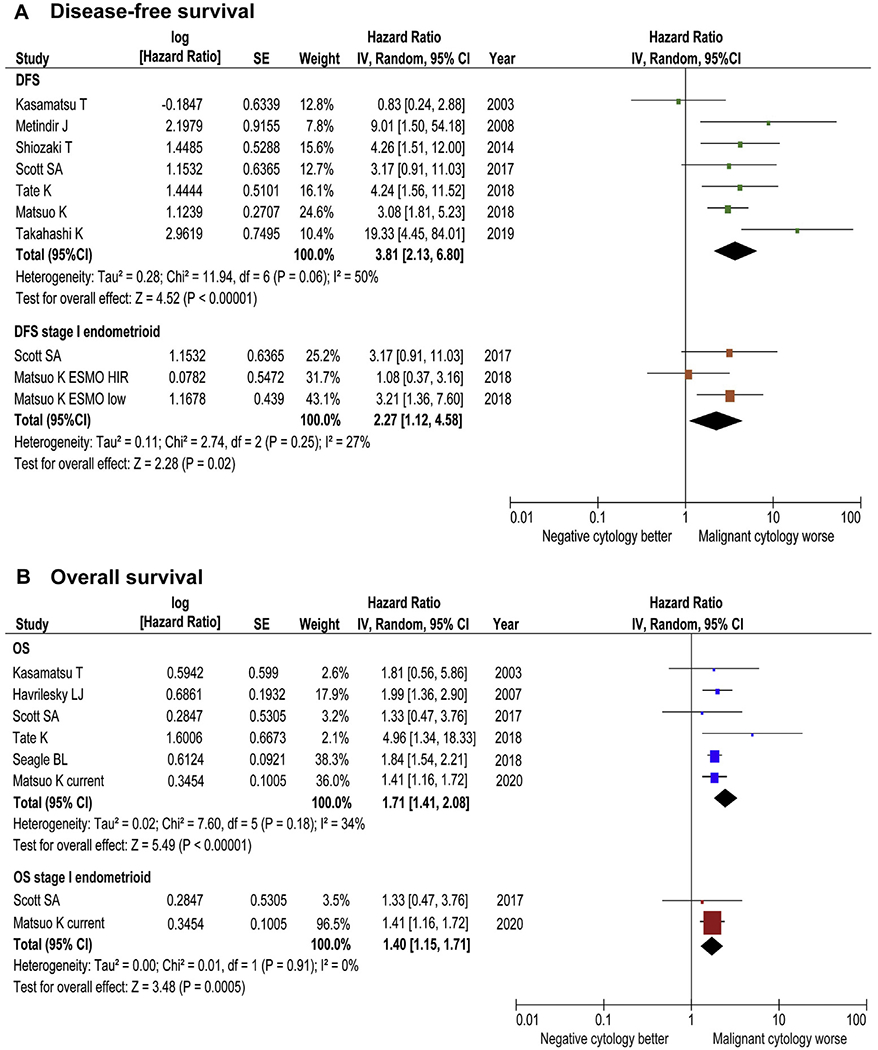
Results of meta-analysis are shown for (A) DFS and (B) OS. Funnel plots are shown in Fig. S6. A forest plot from a random effects meta-analysis of seven studies for DFS and six studies for OS (including our study) stratified by inclusion criteria and ordered within stratum by year of publication and relative weight (%) of study. Heterogeneity was low to moderate across on disease-free survival (A, DFS: I2 = 50%, stage I endometrioid: I2 = 27%) and low across studies on overall survival (B, OS: I2 = 8%, stage I endometrioid: I2 = 0%). DFS, disease-free survival; OS, overall survival.
4. Discussion
Key findings of the present study are that (i) malignant peritoneal cytology was seen in ~4% of stage I endometrioid endometrial cancer and (ii) malignant peritoneal cytology is associated with a twofold increased risk of endometrial cancer death and approximately 40% increased risk of all-cause mortality in stage I endometrioid endometrial cancer. The absolute survival rate difference was more pronounced in the high-–intermediate-risk group.
The presence of malignant peritoneal cytology in stage I endometrioid endometrial cancer is not rare (~4%), which is comparable to other tumour factors in stage I endometrial cancer. For instance, lymphovascular space invasion (LVSI), another key tumour factor, is seen in ~6% of stage I endometrial cancer [37]. Therefore, awareness of malignant peritoneal cytology as a prevalent tumour factor in early-stage endometrial cancer is necessary.
This study suggests that malignant peritoneal cytology is a prognostic factor in stage I endometrioid endometrial cancer. Malignant peritoneal cytology was associated with other aggressive tumour factors such as deep myometrial tumour invasion and higher tumour grades. Thus, one may argue that decreased survival outcome related to malignant peritoneal cytology may be due to these other tumour factors. Nevertheless, subgroup analysis of the high–intermediate-risk group clearly supports malignant peritoneal cytology as an independent prognostic factor for poorer oncologic outcome. The absolute 5-year survival rate difference of 9.7% for CSS in this group is clinically significant. Collectively, incorporating malignant peritoneal cytology status into prognosis and treatment algorithms may be necessary.
A prior study showed that chemotherapy may possibly reduce peritoneal dissemination for early-stage endometrial cancer with malignant peritoneal cytology [10], and this topic requires further attention. The current standard modality for postoperative treatment is local radiotherapy but not systemic chemotherapy in patients with high–intermediate-risk endometrioid endometrial cancer [4,5]. Neither of the two recent phase III trials examined the impact of malignant peritoneal cytology in survival stratification and treatment outcome (GOG-249 and PORTEC-3) [38,39]. It would be of interest if treatment responses differed between chemotherapy-based and radiotherapy-based approaches when stratified by the peritoneal cytology status in these trials. The present study also suggests the potential benefit of adjuvant chemotherapy in cases with malignant peritoneal cytology among the high–intermediate-risk group, but, as the sample size is limited, additional external validation is necessary to confirm this association.
The pathophysiology of malignant peritoneal cytology in endometrial cancer has not been completely delineated, but, a recent study (GOG-210) found that tubal ligation is protective against malignant peritoneal cytology in endometrial cancer (risk reduction >80%) [40]. A similar association was observed for advanced-stage tumours. These findings imply that one possible mechanism for the presence of malignant cells in the peritoneal cavity is metastasis through the fallopian tubes via retrograde tumour spread. To date, the association between hysteroscopic endometrial sampling and endometrial cancer survival is controversial, and this area merits additional studies [41,42].
The association between performance of lymphadenectomy and increased incidence of malignant peritoneal cytology is unexplained in this study. It is likely that there were unmeasured factors that confounded the results. Potential confounders may include lymphadenectomy type (minimally invasive versus laparotomy), surgeon’s preference for preoperative hysteroscopy use and institutional diagnostic criteria for malignant peritoneal cytology. This information was not available for this study, and further investigation is warranted.
A strength of the study is that it is population-based with the largest sample size in the literature so far. PS-IPTW analyses, various sensitivity analyses and a systematic review with meta-analysis enriched the statistical rigour.
There are also multiple limitations in this study. First, unmeasured bias is inherent in any retrospective study. For instance, details of surgical–pathological factors such as LVSI, presence of lower uterine segment involvement and peculiar pattern of invasion were not available, but may have been unbalanced between the two groups and impacted survival outcome [43]. The high–intermediate-risk group was therefore not assessable by the GOG-99 criteria [44]. As noted above, significant factors such as a personal history of tubal surgery as well as use of hysteroscopic surgery were not available in the database and may have confounded the study results if the common cause of malignant peritoneal cytology was, for example, the absence of tubal surgery and the presence of hysteroscopic surgery.
Second, the follow-up duration was relatively short, so the study may be susceptible to a lead-time bias. Third, anatomical recurrent sites were not available in the database, and it is unknown if malignant peritoneal cytology is associated with distant or peritoneal recurrence. Fourth, utility of molecular classification has been proposed in endometrial cancer, but the SEER program does not have this information [21]. Fifth, sample size remained limited for the malignant peritoneal cytology cases in high–intermediate-risk group. Last, no central pathology review was performed for peritoneal cytology, and diagnostic criteria and the accuracy for malignant peritoneal cytology were unknown.
5. Conclusion
The present study suggests that malignant peritoneal cytology is associated with decreased survival in women with stage I endometrioid endometrial cancer. According to our review of the literature, the significance of malignant peritoneal cytology has been understudied in stage I endometrioid endometrial cancer, but our meta-analysis supports the findings of our population-based study. No prospective interventional study has so far examined the prognostic impact of malignant peritoneal cytology in early-stage endometrial cancer. GOG-210 prospectively collected information for malignant peritoneal cytology, but the study was not early-stage specific [22]. Thus, large-scale prospective studies are warranted to confirm our study findings in early-stage endometrial cancer. Based on our findings, peritoneal washings should be performed even in early-stage endometrioid endometrial cancer patients. Utility of malignant peritoneal cytology as the possible source of biomarker for tumour exosomes and circulating tumour DNA is currently under active investigation and warrants further development [45].
Supplementary Material
Acknowledgments
Funding support
Ensign Endowment for Gynecologic Cancer Research (K.M.).
Role of funding for the study
None.
Conflict of interest statement
Consultant, Tesaro and Clovis Oncology, research funding, Merck (J.D.W.); consultant, Quantgene (L.D.R.); honorarium, Chugai, textbook editorial expense, Springer and investigator meeting attendance expense, VBL therapeutics (K.M.); research funding, MSD (S.M.); personal fees, non-financial supports, honoraria for lectures, advisory board and travel support, AstraZeneca and Roche, non-financial support and travel support, Medac, personal fees, honoraria for lectures and advisory board, Tesaro and Pharmamar, personal fees and honoraria for advisory board, Clovis and Lilly, personal fees and honoraria for lectures, Stryker, personal fees and honoraria for advisory board, Immunogen (H.P.); advisory board, Tesaro, GSK (M.K.); none for others.
Footnotes
Ethical committee exemption
University of Southern California (HS-16-00481).
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2020.03.031.
References
- [1].Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet (Lond Engl) 2016;387:1094–108. [DOI] [PubMed] [Google Scholar]
- [3].Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet (Lond Engl) 2012;379:1352–60. [DOI] [PubMed] [Google Scholar]
- [4].Uterine Neoplasms. NCCN clinical practice guidelines in oncology. National Comprehensive Cancer Network; https://www.nccn.org/professionals/physician_gls/. [Accessed 17 January 2020]. [Google Scholar]
- [5].Meyer LA, Bohlke K, Powell MA, et al. Postoperative radiation therapy for endometrial cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol – Off J Am Soc Clin Oncol 2015;33:2908–13. [DOI] [PubMed] [Google Scholar]
- [6].Pecorelli S Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet – Off Organ Int Feder Gynaecol Obstet 2009;105:103–4. [DOI] [PubMed] [Google Scholar]
- [7].Tebeu PM, Popowski Y, Verkooijen HM, et al. Positive peritoneal cytology in early-stage endometrial cancer does not influence prognosis. Br J Cancer 2004;91:720–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kasamatsu T, Onda T, Katsumata N, et al. Prognostic significance of positive peritoneal cytology in endometrial carcinoma confined to the uterus. Br J Cancer 2003;88:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Takahashi K, Yunokawa M, Sasada S, et al. A novel prediction score for predicting the baseline risk of recurrence of stage I-II endometrial carcinoma. J Gynecol Oncol 2019;30:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Matsuo K, Yabuno A, Hom MS, et al. Significance of abnormal peritoneal cytology on survival of women with stage I-II endometrioid endometrial cancer. Gynecol Oncol 2018;149:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tate K, Yoshida H, Ishikawa M, et al. Prognostic factors for patients with early-stage uterine serous carcinoma without adjuvant therapy. J Gynecol Oncol 2018;29:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Seagle BL, Alexander AL, Lantsman T, Shahabi S. Prognosis and treatment of positive peritoneal cytology in early endometrial cancer: matched cohort analyses from the National Cancer Database. Am J Obstet Gynecol 2018;218 329.e1–329.e15. [DOI] [PubMed] [Google Scholar]
- [13].Scott SA, van der Zanden C, Cai E, McGahan CE, Kwon JS. Prognostic significance of peritoneal cytology in low-intermediate risk endometrial cancer. Gynecol Oncol 2017;145:262–8. [DOI] [PubMed] [Google Scholar]
- [14].Shiozaki T, Tabata T, Yamada T, Yamamoto Y, Yamawaki T, Ikeda T. Does positive peritoneal cytology not affect the prognosis for stage I uterine endometrial cancer?: the remaining controversy and review of the literature. Int J Gynecol Cancer – Off J Int Gynecol Cancer Soc 2014;24:549–55. [DOI] [PubMed] [Google Scholar]
- [15].Haltia UM, Butzow R, Leminen A, Loukovaara M. FIGO 1988 versus 2009 staging for endometrial carcinoma: a comparative study on prediction of survival and stage distribution according to histologic subtype. J Gynecol Oncol 2014;25:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Garg G, Gao F, Wright JD, Hagemann AR, Mutch DG, Powell MA. Positive peritoneal cytology is an independent risk-factor in early stage endometrial cancer. Gynecol Oncol 2013;128:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bansal S, Buck AM, Herzog TJ, Deutsch I, Burke WM, Wright JD. Stage IIIA endometrial carcinoma: outcome and predictors of survival. Obstet Gynecol 2009;114:100–5. [DOI] [PubMed] [Google Scholar]
- [18].Metindir J, Bilir Dilek G. Positive peritoneal cytology and its prognostic value in endometrioid adenocancer confined to the uterus. Oncologie 2008;10:348–51. [Google Scholar]
- [19].Havrilesky LJ, Cragun JM, Calingaert B, et al. The prognostic significance of positive peritoneal cytology and adnexal/serosal metastasis in stage IIIA endometrial cancer. Gynecol Oncol 2007;104:401–5. [DOI] [PubMed] [Google Scholar]
- [20].Saga Y, Imai M, Jobo T, et al. Is peritoneal cytology a prognostic factor of endometrial cancer confined to the uterus? Gynecol Oncol 2006;103:277–80. [DOI] [PubMed] [Google Scholar]
- [21].Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Creasman WT, Ali S, Mutch DG, et al. Surgical-pathological findings in type 1 and 2 endometrial cancer: an NRG Oncology/Gynecologic Oncology Group study on GOG-210 protocol. Gynecol Oncol 2017;145:519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].National Cancer Institute’s. The surveillance, epidemiology, and end results (SEER) program. http://seer.cancer.gov/. [Accessed 16 January 2020].
- [24].National Cancer Registrars Association. http://www.ncra-usa.org/i4a/pages/index.cfm?pageid
- [25].National Death Index. Available online: https://www.cdc.gov/nchs/ndi/. [Accessed on 28 June 2019].
- [26].Corpus Carcinoma. CS site-specific factor 2 peritoneal cytology. http://web2.facs.org/cstage0205/corpuscarcinoma/CorpusCarcinoma_kpo.html. [Accessed 18 January 2020].
- [27].Jamison PM, Altekruse SF, Chang JT, et al. Site-specific factors for cancer of the corpus uteri from SEER registries: collaborative stage data collection system, version 1 and version 2. Cancer 2014;120(Suppl. 23):3836–45. [DOI] [PubMed] [Google Scholar]
- [28].Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post operative radiation therapy in endometrial carcinoma. Lancet (Lond Engl) 2000;355:1404–11. [DOI] [PubMed] [Google Scholar]
- [29].Matsuo K, Machida H, Blake EA, et al. Trends and outcomes of women with synchronous endometrial and ovarian cancer. Oncotarget 2018;9:28757–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Voss MA, Ganesan R, Ludeman L, et al. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer-a clinical and pathological evaluation. Gynecol Oncol 2012;124:15–20. [DOI] [PubMed] [Google Scholar]
- [31].Matsuo K, Opper NR, Ciccone MA, et al. Time interval between endometrial biopsy and surgical staging for type I endometrial cancer: association between tumor characteristics and survival outcome. Obstet Gynecol 2015;125:424–33. [DOI] [PubMed] [Google Scholar]
- [32].Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med 1997;16:965–80. [DOI] [PubMed] [Google Scholar]
- [33].Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hershman DL, Wright JD. Comparative effectiveness research in oncology methodology: observational data. J Clin Oncol – Off J Am Soc Clin Oncol 2012;30:4215–22. [DOI] [PubMed] [Google Scholar]
- [35].Cohen J Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- [36].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Matsuo K, Garcia-Sayre J, Medeiros F, et al. Impact of depth and extent of lymphovascular space invasion on lymph node metastasis and recurrence patterns in endometrial cancer. J Surg Oncol 2015;112:669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Randall ME, Filiaci V, McMeekin DS, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol – Off J Am Soc Clin Oncol 2019;37:1810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].de Boer SM, Powell ME, Mileshkin L, et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol 2019;20:1273–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Felix AS, Brinton LA, McMeekin DS, et al. Relationships of tubal ligation to endometrial carcinoma stage and mortality in the NRG oncology/gynecologic oncology group 210 trial. J Natl Cancer Inst 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Polyzos NP, Mauri D, Tsioras S, Messini CI, Valachis A, Messinis IE. Intraperitoneal dissemination of endometrial cancer cells after hysteroscopy: a systematic review and meta-analysis. Int J Gynecol Cancer – Off J Int Gynecol Cancer Soc 2010;20:261–7. [DOI] [PubMed] [Google Scholar]
- [42].Chang YN, Zhang Y, Wang YJ, Wang LP, Duan H. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril 2011;96:957–61. [DOI] [PubMed] [Google Scholar]
- [43].Joehlin-Price AS, McHugh KE, Stephens JA, et al. The microcystic, elongated, and fragmented (MELF) pattern of invasion: a single institution report of 464 consecutive FIGO grade 1 endometrial endometrioid adenocarcinomas. Am J Surg Pathol 2017;41:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2004;92:744–51. [DOI] [PubMed] [Google Scholar]
- [45].Mayo-de-Las-Casas C, Velasco A, Sanchez D, et al. Detection of somatic mutations in peritoneal lavages and plasma of endometrial cancer patients: a proof-of-concept study. Int J Cancer 2020. July 1;147(1):277–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


