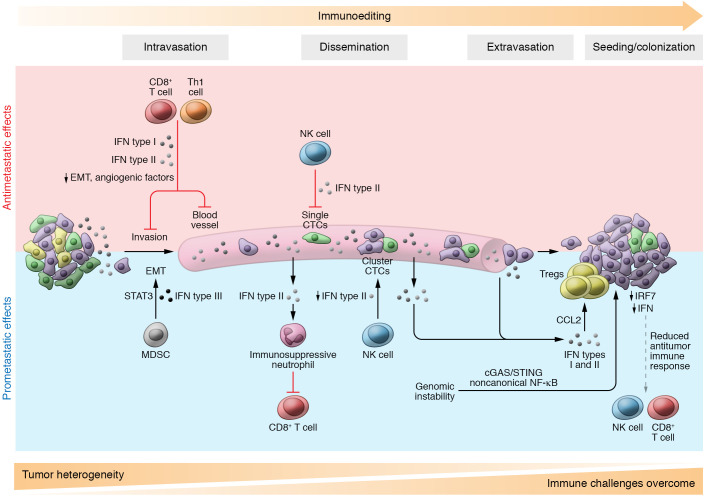
Figure 3. IFN effects during metastasis.
Antimetastatic effects: IFNs might reduce tumor cell dissemination through upregulation of E-cadherin by IFN type I, thus inhibiting epithelial-mesenchymal transition (EMT). Also, CD8+ T cells and Th1 cells secrete IFN type II, which downregulates both CXCR4 and VEGF, suppressing dissemination and angiogenesis, respectively. Upon dissemination, EMT-like single circulating tumor cells (CTCs) are susceptible to NK cell–mediated killing, while CTC clusters contain epithelial-like cells that are less susceptible to NK cell–mediated cytotoxicity, causing reduced IFN-γ production by NK cells. At the metastatic site, tumors display reduced IRF7 expression, diminishing IFN and visibility to CD8+ T and NK cell immune attack. Prometastatic effects: Myeloid-derived suppressor cells (MDSCs) release IFN type III, which activates STAT3, engaging the EMT process. IFN types I and II are produced by tumor cells, driving the recruitment of immunosuppressive neutrophils that decrease immune attack during dissemination. Also, IFN types I and II lead to CCL2 secretion and increase recruitment of Tregs to the metastatic site, supporting the seeding of tumor cells. Genomic instability triggers cGAS/STING pathways, promoting invasion and metastasis. The dynamic interaction with immunity could be the cause of tumor heterogeneity loss and the increase in clonal tumor selection driven by IFN sensitivity. Immune hostile challenges accumulate throughout the process, contributing to immunoediting.