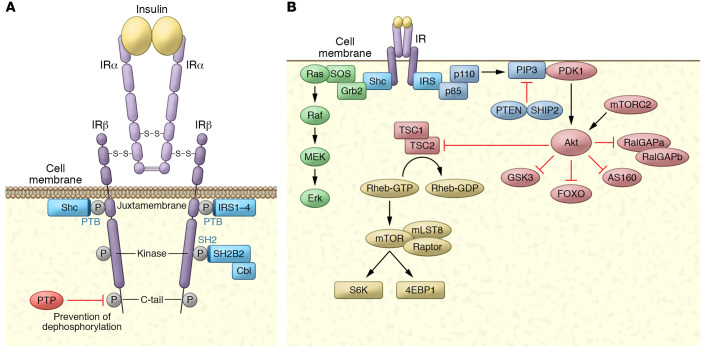
Figure 1. The insulin receptor, its substrates, and its activation of kinase cascades.
(A) The insulin receptor is a disulfide-linked, α/β heterodimer glycoprotein that resides largely on the cell surface. The α subunit binds to insulin with high affinity, alleviating PTP-mediated repression of the β subunit’s tyrosine kinase activity by inducing close proximity between the β subunits, permitting transphosphorylation on tyrosines in three β subunit domains. Phosphorylation of three crucial tyrosines leads to full activation of the receptor kinase. Once activated, the receptor kinase can phosphorylate exogenous substrates that act as adaptors: IRS-1–IRS-4 and Shc. Both are recruited to the juxtamembrane region via their PTB domains. SH2B2 is recruited to the kinase region’s triple phosphorylation motif via its SH2 domain, serving as an adaptor protein for the substrate Cbl. (B) Activation of kinase cascades. Once phosphorylated, IRS and Shc activate lipid and protein kinases. IRS proteins are phosphorylated on tyrosines within specific motifs, recruiting the p85 subunit of PI3K, which binds to IRS through its SH2 domain. This results in activation of the p110 catalytic domain to generate polyphosphoinositides such as PI-(3,4,5)trisphosphate (PIP3). These phosphoinositides can be degraded by the PI phosphatases PTEN and SHIP2. PIP3 interacts with proteins containing PH domains, notably PDK1 and Akt. Once recruited to the plasma membrane, PDK1 and mTORC2 phosphorylate and activate Akt, which can phosphorylate a number of substrates, including the GAP proteins RalGAPA, AS160, and TSC2, as well as Foxo proteins, GSK3, and others. Upon phosphorylation, Shc interacts with the SH2/SH3 adaptor protein Grb2, which is constitutively associated with the GEF SOS. SOS is thus recruited to the plasma membrane, and catalyzes the exchange of GTP for GDP on Ras. In its active, GTP-bound state, Ras interacts with the protein kinase Raf, leading to activation of the MAPK cascade through sequential phosphorylation of MEK and ERK.