Figure 2. Regulation of glycogen metabolism by compartmentalized phosphorylation.
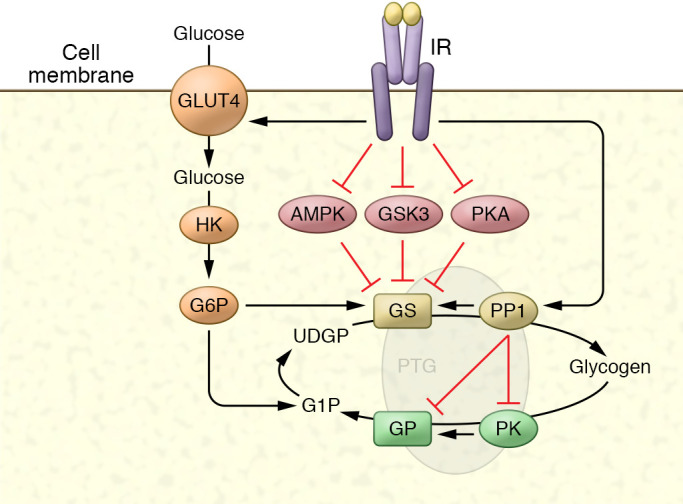
Like other metabolic enzymes, control of glycogen metabolism is mediated by changes in phosphorylation of the enzymes glycogen synthase (GS) and glycogen phosphorylase (GP) through inhibition of kinases and activation of phosphatases. GS is inhibited by phosphorylation on up to nine amino acids, and insulin activates the enzyme by reversing this phosphorylation through a combination of kinase inhibition and phosphatase activation, primarily through protein phosphatase 1 (PP1). Similarly, GP is activated by phosphorylation, and insulin inhibits the enzyme by reducing phosphorylation. These events occur in discrete cellular compartments owing to the presence of scaffolding proteins such as PTG (Ppp1R3C) and others, by binding to GS, GP, phosphorylase kinase (PK), and AMPK, and targeting these proteins to glycogen itself. GS is also regulated by the binding of glucose-6-phosphate (G6P) to an allosteric site that increases activity.
