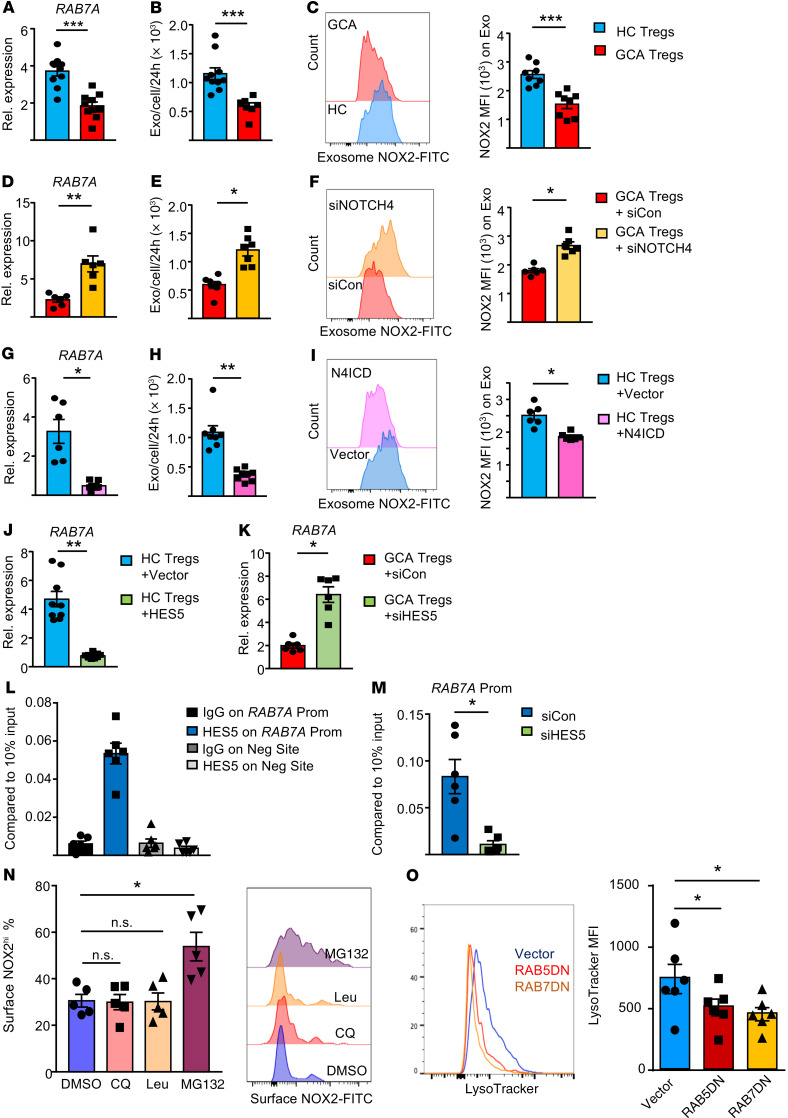
Figure 7. NOTCH4 signaling regulates exosome production.
(A) Gene expression for the late endosome marker RAB7A in CD8+ Tregs (RT-PCR, n = 10). (B) Quantification of CD8+ Treg–produced exosomes (n = 10 controls, 7 patients). (C) NOX2 protein expressed on secreted exosomes. Representative histograms and FACS results from 8 samples each. (D–F) GCA CD8+ Tregs were transfected with NOTCH4 or control siRNA. (D) RAB7A transcripts. RT-PCR from 6 patients. (E) Secreted exosomes from n = 7 samples. (F) Exosomal NOX2 protein. Flow cytometry from 6 samples. (G–I) Healthy CD8+ Tregs were transfected with an N4ICD plasmid or empty vector. (G) RAB7A transcripts quantified by RT-PCR in 6 samples. (H) Secreted exosomes determined in 8 samples. (I) Exosomal NOX2 protein. Flow cytometry from 6 samples. (J and K) Control CD8+ Tregs (J) and GCA CD8+ Tregs (K) were transfected as indicated. (J) HES5-containing or empty vector; (K) HES5 or control siRNA. RT-PCR for RAB7A transcripts in n = 6 samples. (L) ChIP assays targeting HES5 or control IgG were performed on the promoter of RAB7A or a negative site. Signal normalized to 10% of input. Data from 6 GCA CD8+ Treg samples. (M) GCA CD8+ Tregs were transfected with HES5 or control siRNA. Occupancy of HES5 on the RAB7A promoter was examined by ChIP assay. Signal normalized to 10% of input. n = 6 samples. (N) GCA CD8+ Tregs were treated with the lysosomal inhibitors chloroquine (CQ) and leupeptin (Leu), the proteasome inhibitor MG132, or vehicle. Cell surface NOX2 was evaluated by FACS. Representative histograms and results from 5 samples. (O) GCA CD8+ Tregs were transfected with a RAB5DN-containing, a RAB7DN-containing, or control vector. Lysosome intensity was measured by LysoTracker. Representative histograms and FACS results from 6 samples. CD8+ Treg cells were induced ex vivo. Data are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 by unpaired (A–C) and paired (D–K and M) Mann-Whitney-Wilcoxon rank test, or ANOVA and post-ANOVA pairwise 2-group comparisons conducted with Tukey’s method (N and O).