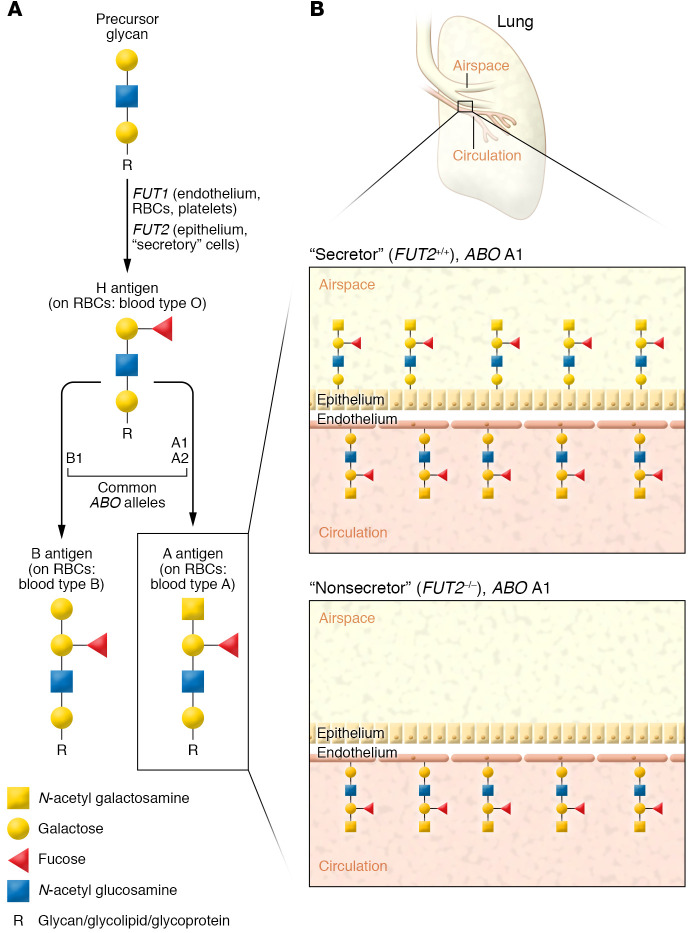
Figure 1. Cell-specific expression of ABH antigens.
(A) Cell type–specific fucosyltransferases (FUT1 in multiple cell types; FUT2 in epithelial and secretory cells) create H antigens by adding a fucose monosaccharide to the nonreducing (free) ends of glycans anchored to glycolipids or glycoproteins. H antigens are then modified by glycosyltransferases encoded by the ABO genes. ABO allele A1 produces a highly active glycosyltransferase that imparts high A antigen density on cell surfaces. ABO allele O produces a nonfunctional glycosyltransferase, leaving the H antigen unmodified. A, B, or H antigens on red blood cells (RBCs) impart blood types A, B, or O, respectively. Simplified schema does not show nature of glycan linkages (i.e., α2 linkage of fucose). (B) Approximately 20% of European populations have loss-of-function mutations in FUT2, leading to an inability to form H antigens (and consequently, A or B antigens) on epithelial surfaces. As such, FUT2–/– individuals with ABO A1 alleles will express a high density of A antigens on erythrocytes and endothelial cells but have no expression of A antigens on epithelial surfaces or mucosal secretions. Simplified schema excludes underlying glycan core structures. A antigens can be further modified into hyperglycosylated A1 and A2 antigen subtypes; only A antigens are shown for simplicity.