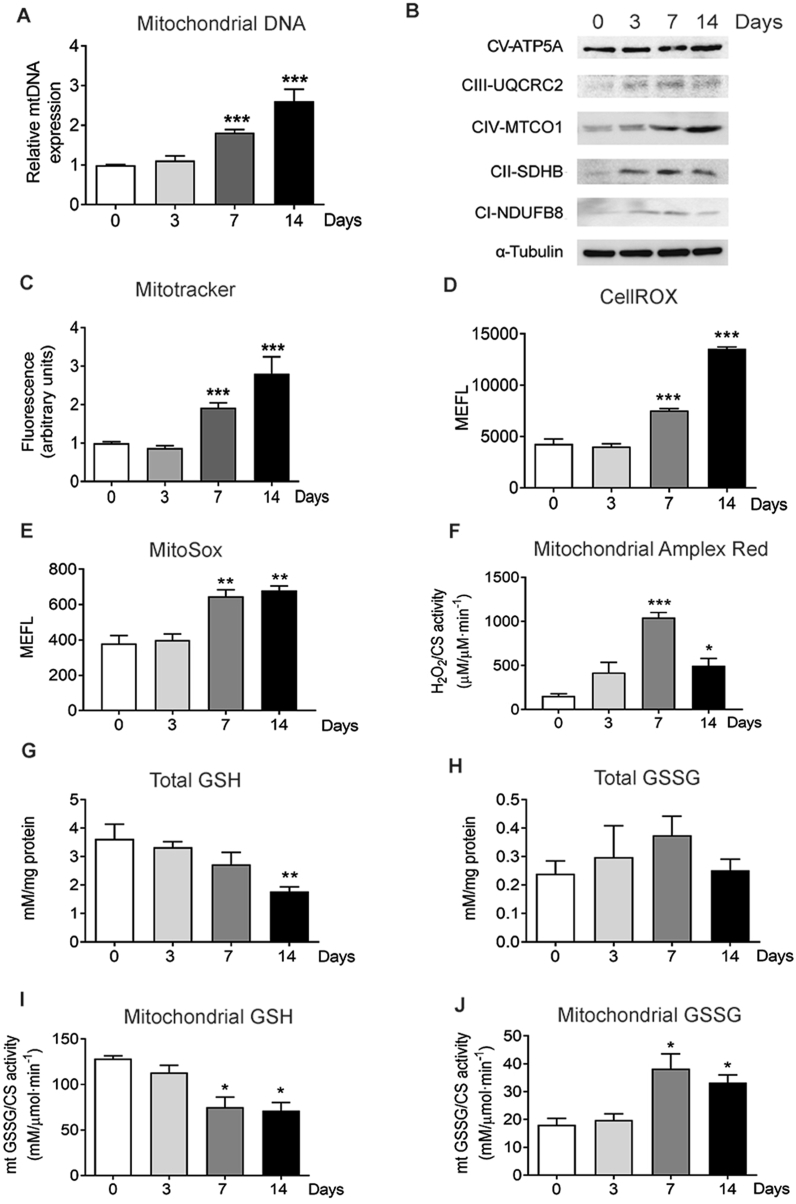
Fig. 1.
Increased OXPHOS activity and ROS generation are associated with osteocytic differentiation. (A) Quantification of mtDNA in undifferentiated IDG-SW3 after 3, 7, and 14 days of differentiation. Results are plotted as expression relative to undifferentiated IDG-SW3 (mean ± SEM; n = 7). (B) Analysis of the levels of mitochondrial complexes in undifferentiated IDG-SW3 after 3, 7, and 14 days of differentiation (C) Flow cytometry analysis of mitochondrial membrane potential using Mitotracker Deep Red in undifferentiated IDG-SW3 and after 3, 7, and 14 days of differentiation. Results are plotted as expression relative to undifferentiated IDG-SW3 (mean ± SEM; n = 8). (D) Flow cytometry analysis of total ROS stained with CellROX Deep Red in undifferentiated IDG-SW3 and after 3, 7, and 14 days of differentiation. Results are plotted as Molecules of Equivalent Fluorescein (mean ± SEM; n = 4). (E) Flow cytometry analysis of mitochondrial superoxide stained with MitoSOX Red in undifferentiated IDG-SW3 and after 3, 7, and 14 days of differentiation. Results are plotted as Molecules of Equivalent Fluorescein (mean ± SEM; n = 4). (F) Determination of superoxide produced in isolated mitochondria using Amplex Red. Results were normalized to citrate synthase (CS) activity. (mean ± SEM; n = 3). (G and H) Total GSH (G) and GSSG (H) in undifferentiated IDG-SW3 and after 3, 7, and 14 days of differentiation. Results are plotted as mean ± SEM (n = 5). (I, and J) GSH (I) and GSSG (J) levels in isolated mitochondria from undifferentiated IDG-SW3 and after 3, 7, and 14 days of differentiation. Results were normalized to citrate synthase (CS) activity (mean ± SEM; n = 4 *p < 0.05, **p < 0.01, and ***p < 0.001 using Student's t-test.