Abstract
Background
Postmenopausal women exhibit higher rates of disability and cardiovascular disease (CVD) with aging compared to men. Whereas habitual exercise training is a known strategy to enhance physiologic function in men and premenopausal women, exercise-related adaptations are often modest in postmenopausal women. We propose dietary nitrate (beetroot juice) administered prior to exercise training may be a feasible approach to improve mobility and cardio-metabolic health outcomes in postmenopausal women.
Methods
Our randomized, placebo-controlled study aims to determine preliminary effects sizes for changes in functional mobility and endothelium-dependent vasodilation across three study arms: exercise only (EX), exercise + placebo (EX + PL), and exercise + beetroot (EX + BR). Thirty-six postmenopausal women are recruited in small cohorts wherein group exercise is implemented to facilitate social support and adherence to an 8-week training progression. Participants are randomized to one of three study arms (n = 12 per group) following baseline assessments. Post-intervention assessments are used to determine pre-post changes in outcome measures including distance covered during a 6 min walk test, walking economy, muscle speed and power, and endothelial-dependent vasodilation as determined by flow-mediated dilation. Measures of feasibility include recruitment, retention, adherence to exercise prescription, perceived exercise session difficulty, and adverse event rates.
Discussion
Evidence-based, translational strategies are needed to optimize exercise training-related adaptations in postmenopausal women. Findings will inform larger randomized clinical trials to determine if pre-exercise consumption of beetroot juice is an efficacious strategy to promote mobility and attenuate CVD disease risk.
Keywords: Dietary nitrate, Nitric oxide, Postmenopausal, Menopause, Cardiovascular disease, Vascular health
1. Introduction
The principle age-related barrier to healthy, independent living is a progressive decline in physiologic function and movement quality. Thus, the preservation of health and quality-of-life are of significance to over 50 million individuals aged 65 years and older in the United States [1]. Postmenopausal women, in particular, represent a growing segment of the population that exhibit higher rates of disability and cardiovascular disease (CVD) with advancing age compared to men [2,3]. While habitual exercise training is an effective strategy to slow age-related decrements in physiologic function, strong evidence suggests the vascular adaptations (i.e., enhanced vasodilator capacity of skeletal muscle microvasculature) to training are dependent on sex and reproductive age [4,5]. Postmenopausal women do not consistently possess the same vascular responsiveness to exercise compared to premenopausal women [6] or men [7]. As such, targeted efforts are needed to overcome the limitations of current exercise prescriptions to attenuate age-related shifts in CVD risk and loss of functional mobility among postmenopausal women.
A combination of age-related oxidative stress and diminishing endogenous estrogen [8,9] likely contribute to an increased CVD risk and attendant nitric oxide insufficiency among postmenopausal women. Since nitric oxide is essential for numerous physiologic functions, including endothelial-dependent vasodilation, inhibition of platelet aggregation, and muscular efficiency/contractility [10,11], declining nitric oxide bioavailability is implicated in vascular endothelial dysfunction and an impaired adaptive response to exercise training stimuli [12,13]. Exogenous estrogen treatments are a seemingly plausible remedy to restore physiologic function in postmenopausal women, as estrogen is directly implicated in nitric oxide biosynthesis [14] and exerts cardioprotective benefits in premenopausal women [15]. However, data from the Women's Health Initiative and the Heart and Estrogen/Progestin Replacement Study does not support the use of estrogen replacement therapy for the long-term prevention of CVD risk after menopause [15,16]. Therefore, it is essential to consider alternative approaches that increase nitric oxide bioavailability to improve mobility and vascular health in postmenopausal women.
In response, a three-arm randomized, placebo-controlled trial was developed to test the feasibility of a dietary nitrate supplement (in the form of beetroot juice) administered prior to exercise with an overall objective seeking to augment the benefits of habitual exercise training. While historically the oxidation of endogenous l-arginine was thought to be the primary source of nitric oxide production, recently an enterosalivary circuit has been identified wherein ingested nitrate (from, e.g., leafy greens, beetroot) are reduced to nitrite by bacteria in the oral cavity and subsequently to nitric oxide in systemic circulation [17]. Compelling evidence in humans have shown reduced blood pressure [18,19], enhanced endothelial-dependent vasodilation [20] and improved exercise performance [21] with dietary nitrate supplementation. However, the feasibility of dietary nitrate supplementation combined with habitual exercise training is largely unknown in older populations. Thus, the ongoing BEE SWEET project aims to test the feasibility of pre-exercise beetroot juice supplementation and to determine preliminary effect sizes for outcome measures of functional mobility and endothelial-dependent vasodilation to inform a larger-scale randomized clinical trial.
2. Research goals (specific aims and hypotheses)
The primary aim of the BEE SWEET study is to determine the feasibility of an 8 week exercise training program (EX) coupled with the consumption of beetroot juice (EX + BR) or placebo (EX + PL) prior to exercise in postmenopausal women. Prior work supports daily, short-term (i.e., 21 days) consumption of beetroot juice in men and women aged 62 ± 1 years [22], yet this was performed in the absence of exercise training. Research unequivocally supports the utility of exercise training in older adults, still in the context of this design, feasibility is unknown. Outcomes of interest include recruitment, retention, adherence to exercise prescription and assessments, perceived difficulty of exercise sessions, and adverse event rates. Project success will be based on 83% [30 of 36] of randomized participants completing 8 weeks of resistance training exercise with analyzable measures for the 6 min walk test, treadmill walking economy, isokinetic dynamometry, endothelium-dependent vasodilation, and blood samples for measures of soluble endothelial microparticles and plasma nitrate/nitrite at baseline and follow-up.
Secondary aims are to determine preliminary effect sizes for EX + BR compared to EX + PL and EX on distance covered during a 6 min walk test, treadmill walking economy, peak power and velocity assessed with isokinetic dynamometry, and endothelial-dependent vasodilation as evidenced by percent increase in flow-mediated dilation and cutaneous vascular conductance. Consistent with others showing performance benefits of beetroot juice consumption during aerobic exercise performance [23] and endothelial function [24], we anticipate a small (η2 = 0.01) to medium (η2 = 0.06) effect size in a beneficial direction for EX + BR compared to EX + PL and EX. Further information related to effect size interpretation is described by Lakens (2013) [25].
An exploratory aim is to determine the preliminary effect size for EX + BR compared to EX + PL and EX for changes in soluble endothelial microparticles including soluble vascular cell adhesion molecule-1 (sVCAM-1), soluble intracellular adhesion molecule-1 (sICAM-1), and soluble E-selectin. Given the link between nitric oxide bioavailability and regulation of NF-ҡB [26], it is reasonable that EX + BR may have synergistic properties that could elicit favorable effects on the vascular endothelium. As such, we anticipate a small to medium effect size [η2 = 0.01–0.06 [25]] in a beneficial direction for EX + BR compared to EX + PL and EX.
3. Study design
3.1. Rationale for the use of beetroot juice (dietary nitrate)
It is understood that age-related oxidative stress contributes to nitric oxide insufficiency [9]. In this context, the formation of peroxynitrite, attributable to the interaction between nitric oxide and superoxide, lowers nitric oxide bioactivity and triggers local injury including cellular necrosis/apoptosis [27]. Since nitrate and nitrite can be reduced to form nitric oxide, the nitrate-nitrite-nitric oxide pathway represents an alternative route to enhance the bioavailability of nitric oxide at the tissue level [28,29]. Supplementation with dietary nitrate, a naturally occurring compound in leafy greens and beetroot, is one such strategy to enhance nitric oxide bioavailability in populations with nitric oxide insufficiencies [30,31]. In populations with impaired endogenous nitric oxide synthesis, acute dietary nitrate supplementation has resulted in lowered peripheral resistance [32], greater skeletal muscle oxygenation [33], improved exercise tolerance [30], enhanced muscle contractility [34,35] and more robust vascular responses to exercise training [31].
Since peak plasma concentrations of nitrate and nitrite occur within 1–2 h and 3–4 h following ingestion of dietary nitrate [36], respectively, there is a reasonable window of time to perform exercise. Since deoxyhemoglobin can trigger the one-electron reduction of nitrite to nitric oxide, often accelerated in conditions of low oxygen tension and pH [37], it is likely ample nitrite to nitric oxide conversion will occur in active skeletal muscle [23,38]. By harnessing the practical time (2–3 h), during which plasma nitrate and nitrite levels are elevated, beetroot juice consumption can be planned to coincide with the start of exercise. This, in turn, will acutely elicit the following effects in postmenopausal women: 1) improved blood flow/muscle oxygenation; 2) greater exercise tolerance and; 3) lower perceived exercise difficulty.
3.2. Study population
A total of 36 postmenopausal women are randomized to one of three interventions: 1) EX (n = 12), 2) EX + PL (n = 12) or, 3) EX + BR (n = 12). Eligible participants are being enrolled at the coordinating center, Indiana University School of Public Health – Bloomington. The objective is to recruit four cohorts of nine women to facilitate adherence through social support with group exercise. Based on 2010 United States Census Bureau Circular Area Profile data taken from a 50 mile radius of the Bloomington testing site, it is anticipated that approximately 83.6% (n = 30) of the sample population will self-identify as White, 9.4% (n = 3) as Black or African American, 2% (n = 1) as Asian, and 4.7% (n = 2) as other or multiple races [39]. Approval from the local Institutional Review Board has been obtained and participants are providing informed consent prior to enrollment.
3.3. Eligibility criteria
The following eligibility criteria are employed to identify postmenopausal women who are between the ages of 55–74 years old and would be able to safely participate in our exercise intervention. Women are included if: 1) had a measured body mass index between 27.0 and 34.9 kg/m2, 2) self-reported as postmenopausal as evidenced by not having a menstrual cycle within the past 12 months, 3) are able to ambulate without assistance, and 4) have physician's clearance to begin an exercise program. Participants are excluded if: 1) currently smoke or use tobacco products, 2) have greater than stage II hypertension (i.e., 159/99 mm Hg), 3) use prescription anti-coagulant medications (i.e., Coumadin or Warfarin), 4) use medication with potent vasodilator effects (i.e., phosphodiesterase-5 inhibitors, calcium channel blockers, nitrates), 5) use medication that alters stomach acid production (i.e., proton pump inhibitors), 6) use medication that affects heart rate (i.e., systemic β-adrenergic blockers), 7) are undergoing hormone replacement therapy, 8) have significant orthopedic limitations or other contraindications to strenuous exercise, 9) anticipate elective surgery during the study period, or 10) live > 50 miles from the Indiana University – Bloomington study site. Further exclusion criteria include a history of 1) habitually exercising ≥3 times per week and 2) diagnosis of a major metabolic disease (i.e., Type I diabetes, Type II diabetes, thyroid disorders).
3.4. Recruitment and screening
Postmenopausal women who meet basic eligibility requirements are being recruited within a 50 mile radius of the Bloomington testing site using indirect methods including posted flyers in/around campus buildings, health clinics, gynecology offices, and local aging organizations. Scripted emails are also sent to campus-wide health and wellness programs (e.g., Healthy IU), local aging group organizations (e.g., Bloomington Commission on Aging, Active Aging Coalition), and faculty list-serves. Study details are available on the Indiana Clinical and Translational Sciences Institute's “All IN for Health” website that matches potentially eligible volunteers to health studies conducted across Indiana and on ClinicalTrials.gov (NCT04370756). Open recruitment occurs for fixed periods of 4–6 weeks.
Recruitment is through self-referral only with potential participants contacting research team members in response to recruitment materials. Those interested undergo a thorough scripted phone screening wherein all study procedures are explained, and a copy of the informed consent statement is sent to the prospective participant via mail or email. Potentially eligible participants are invited to attend an on-site screening that begins with participants providing written informed consent. Eligibility criteria of age (verified by state issued driver ID), prescription medications (verified from current prescription bottles/labels), and measured body mass index are assessed during the on-site screening. Participants are further required to obtain clearance from their primary care physician stating that they are safely able to engage in the outlined exercise protocols.
3.5. Enrollment and randomization
Participants who meet all eligibility requirements and have physician's clearance are enrolled in cohorts of six to nine to facilitate social support and adherence to the supervised exercise training. We anticipate enrolling four waves of nine women to successfully meet the target sample size of 36 postmenopausal women (12 per group).
Group allocation is randomly determined using a computer-based number generator in blocks of three to ensure equal between-group distribution (i.e., n = 1 EX, n = 1 EX + PL, n = 1 EX + BR). For example, in the first wave of nine participants, three are randomly assigned to the EX group, three randomly assigned to the EX + PL group, and three randomly assigned to the EX + BR group. Each assignment is kept in a sealed envelope until completion of all baseline assessment measures.
3.6. Setting
The BEE SWEET project is funded through a Grant Linking University-wide Expertise (GLUE) award sponsored by the Indiana Clinical and Translational Sciences Institute. The mission of GLUE awards is to support collaborative efforts of researchers across Indiana campuses with the goal of developing large multi-investigator projects. Accordingly, outside of the coordinating site at the Indiana University School of Public Health – Bloomington, participants will attend assessment visits at the Clinical Research Center located within the Indiana University Health University Hospital – Indianapolis. Recruitment efforts and enrollment are taking place at the coordinating study site.
3.7. Assessments
3.7.1. Schedule
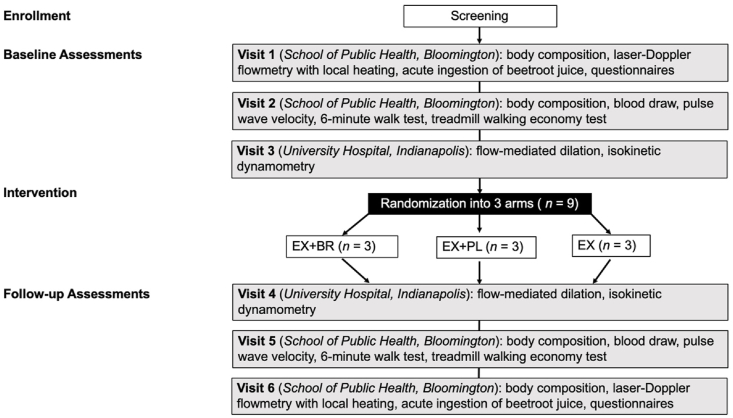
Participants attend three baseline laboratory assessment visits prior to group assignment. Following the 8 week exercise program, baseline testing procedures are repeated across three follow-up assessments. The first assessment visit consists of laser-Doppler flowmetry and measurements of brachial blood pressure completed before and 3 h after acute ingestion of beetroot juice (140 mL); the second, a blood draw followed by pulse-wave velocity analyses, a 6 min walk test and walking economy test performed on a treadmill; and the third, flow-mediated dilation and isokinetic dynamometry. Fig. 1 depicts the visit flow and group randomization whereas Table 1 provides an outline of all measurements taken during the six assessments.
Fig. 1.
Flowchart of assessment visits and intervention randomization for the BEE SWEET trial.
Table 1.
Measures obtained during each assessment visit for the BEE SWEET trial.
| Procedure | Visit 0 Screening | Visit 1 Baseline | Visit 2 Baseline | Visit 3 Baseline | Visit 4 Follow-up | Visit 5 Follow-up | Visit 6 Follow-up |
|---|---|---|---|---|---|---|---|
| Written Informed Consent | X | ||||||
| Medical Health History Questionnaire | X | ||||||
| Obtain Physician Clearance | X | ||||||
| Verification of Eligibility Criteria | X | ||||||
| Body Composition (DEXA) | X | X | |||||
| Body Composition (BIA) | X | X | X | X | |||
| Heart Rate | X | X | X | X | |||
| Blood Pressure | X | X | X | X | |||
| Laser-Doppler Flowmetry | X | X | |||||
| Acute Ingestion of Beetroot Juice (140 mL) | X | X | |||||
| Fractional exhaled nitric oxide | X | X | |||||
| Oxyhemoglobin Saturation/Methemoglobin Oximetry | X | X | |||||
| Questionnaire | X | X | |||||
| Venous Blood Draw | X | X | |||||
| Pulse Wave Velocity Analysis | X | X | |||||
| Treadmill Walking Economy Test | X | X | |||||
| 6 min Walk Test | X | X | |||||
| Accelerometry | X | X | |||||
| Ratings of Perceived Exertion | X | X | |||||
| Flow-Mediated Dilation | X | X | |||||
| Leg Isokinetic Dynamometry | X | X |
3.7.2. Program feasibility measures (primary outcomes)
Recruitment rate will be calculated as the number of participants randomized per months of recruitment time at the coordinating site. The percent of randomized participants with complete outcome measures for the 6 min walk test, walking economy, isokinetic dynamometry, endothelium-dependent vasodilation, and blood samples for concentrations of soluble endothelial microparticles at baseline and follow-up will be calculated as the retention rate.
Adherence to the 8 week exercise program will be assessed as the percent of completed exercise sessions from 24 possible sessions (i.e., three times per week for 8-weeks). Additionally, the number of sessions attended per week (frequency), session heart rate, and rating of perceived exertion (intensity), total minutes of each session (duration), and the number of sets, repetitions, and amount of weight selected for individual exercises performed (volume) will be recorded.
The adherence of participants assigned to the beetroot juice supplementation group will be randomly assessed using the NIOX Vero®(Circassia, Morrisville, NC) for analyses of fractional exhaled nitric oxide concentration 3 h following ingestion. Readings of exhaled nitric oxide will be compared to values obtained during baseline assessment following the acute ingestion of beetroot juice to confirm adherence with supplementation.
The number and frequency of serious adverse events (e.g., chest pain, bone fracture) and minor adverse events (e.g., strained muscle, sore joint) will be monitored.
3.7.3. Measures of functional mobility and endothelial-dependent vasodilation (secondary outcomes)
Consistent with standard practices and procedures [40], participants are instructed to walk for a duration of 6 consecutive minutes around an indoor, closed course. A chest strap heart rate monitor is worn to measure post-exercise heart rate and indices of heart rate recovery at 1, 2, and 3 min following the cessation of walking. A hip-worn triaxial accelerometer is used to examine acceleration signal complexity [41] and estimates of energy expenditure (kilocalories). Participants are asked to identify their level of perceived exertion on a continuous visual analogue scale ranging from “no exertion” to “maximal exertion.” Distance covered during the 6 min walk test is associated with aspects of functional mobility in older adults [[42], [43], [44]]. Acceleration complexity is a measurement of functional capacity that has shown to differentiate between clinical groups, such as older versus young healthy adults and those with an elevated risk for falls [45,46].
Breath-by-breath analyses of oxygen uptake is continuously recorded during treadmill walking at a walking speed of 2 mph and 0% grade for 6 min using a Vmax™ Encore metabolic cart (Vyaire Medical, Mettawa, IL). Resting oxygen uptake, measured for 3 min prior to walking with subjects in a standing position, is subtracted from oxygen uptake averaged across the duration of the walking task to obtain an evaluation of walking economy. Measures of heart rate, blood pressure, and ratings of perceived exertion using the validated Borg CR10 scale [47] are also obtained at minutes three and five of walking task.
Walking self-efficacy is assessed using a validated questionnaire [48] that asks participants to rate their confidence in ability to walk at a moderately-fast pace in incremental 5 min intervals (from 5 to 40 min). Participants rank their confidence on a percentage scale ranging from 0% (“not confident at all”) to 100% (“extremely confident”). A total self-efficacy score is computed by dividing the total sum of all confidence ratings by the number of the items in the scale for an aggregate walking self-efficacy score of 100.
Measures of peak power and velocity of the lower limb are calculated following isokinetic dynamometry using a Biodex System 4 Pro™ (Biodex Medical Systems, Shirley, NY). Briefly, participants perform 3–4 maximal knee extensions with their dominant leg at angular velocities of 0, 1.57, 3.14, 4.71, and 6.28 rad/s with 2 min of rest allowed between each set. As previous described [34], the highest torque generated at each velocity is used to calculate peak power at that velocity, after which the resultant power-velocity curve is fit with a parabolic function to determine peak power and peak velocity (i.e., Y-maximum and 2nd Y-intercept of the fitted parabola), respectively. The intraclass correlations for peak power and peak velocity from our laboratory are 0.98 and 0.94, respectively [49].
Flow-mediated vasodilation is performed by a singular trained vascular technician and used to non-invasively assess in vivo endothelium-dependent vasodilation using a high-resolution (10 MHz), linear-array ultrasound (ACUSON CV70, Siemens Healthcare, Germany) following standardized procedures [50]. Baseline imaging of brachial artery diameter is taken 1–2 cm above the antecubital fossa following 10 min of the participant resting supine in a temperature-controlled room (68–71 °F) with their right arm stabilized by an immobilizer cuff. After baseline measures, a blood pressure cuff placed around the right forearm is inflated to 250 mmHg for 5 min to induce reactive hyperemia. Immediately following deflation of the cuff, at 60 s afterwards, and again at 90 s afterwards, imaging is taken to verify hyperemia. Images are interpreted by a singular, blinded investigator (S.K.G.). The larger percent increase in brachial artery measured at 60 and 90 s is taken as an estimate of flow-mediated vasodilation. The intraclass correlations from our laboratory following these procedures are 0.97 and 0.73 for baseline brachial artery diameter and flow-mediated vasodilation, respectively [51].
Laser-Doppler flowmetry (Moor Instruments, Axminster, UK) is used to assess cutaneous vascular conductance in response to a local heating stimulus. Two small probes seated within heating units are applied to the dorsal aspect of the non-dominant forearm to continuously assess cutaneous red blood cell flux. Beat-to-beat blood pressure is performed using a Finapres® (Finapres Medical Systems, NL). After 5 min of resting measurements, the heating units are warmed at a rate of 1 °C/sec to a set temperature of 39 °C for 25 min. This temperature was selected as it has been shown to be most reflective of nitric oxide dependent vasodilation [52]. The temperature of the heating units is then increased to 44 °C for an additional 15 min. Red blood cell flux and mean arterial pressure are averaged across the final 5 min of heating at 39 °C and 44 °C. Cutaneous vascular conductance at 39 °C is calculated as average red blood cell flux/mean arterial pressure and reported as a percentage of maximum at 44 °C.
Measures of arterial stiffness are performed using pulse wave velocity via ATCOR SphygmoCor® (Naperville, IL). Following 10 min of supine rest, a femoral blood pressure cuff and carotid tonometer are applied simultaneously to obtain pressure waveforms at the carotid and femoral artery sites. SphygmoCor® software uses physical distance measurements between the sites to calculate pulse wave velocity (in milliseconds).
3.7.4. Soluble endothelial microparticle measures (exploratory outcome)
Venous blood samples are drawn by a trained phlebotomist for subsequent analyses of endothelial microparticles. Following centrifugation, samples are aliquoted and kept frozen at −80 °C until batch analyses. The endothelial microparticles of interest, sVCAM-1, sICAM-1, and soluble E-selectin, will be quantified in duplicate using enzyme linked immunosorbent assay technique and spectrophotometry.
3.7.5. Potential covariates
There is a high degree of inter-individual variability in responsiveness to dietary nitrate supplementation [49]. Factors presumably influencing the responsiveness to dietary nitrate include differences in lean tissue mass [53] and nitrate-nitrite-nitric oxide reduction capacity (i.e., oral microbiota, reductase enzymes) [49,54]. To account for potential differences in these factors, total and segmental measures of lean mass and fat mass are obtained at multiple time points across assessment visits using dual-energy X-ray absorptiometry (DEXA; GE Healthcare, Madison, WI) and bioelectrical impedance analysis (BIA; Tanita, Arlington Heights, IL). Additionally, the percent change in fractional exhaled nitric oxide with acute ingestion of the beetroot juice supplement will be determined for each participant. Fractional exhaled nitric oxide non-invasively reflects the reduction of ingested nitrate to nitrite and nitric oxide in the oral cavity [55].
DEXA, dual-energy X-ray absorptiometry. BIA, bioelectrical impedance analysis.
3.8. Interventions
3.8.1. Summary
The BEE SWEET intervention is designed to evaluate an 8 week exercise training program (EX) with the consumption of beetroot juice (EX + BR) or placebo (EX + PL) prior to sessions. Following baseline assessments, cohorts of up to nine women will be randomly assigned to one of three intervention groups. All groups will participate in the exercise training progression.
3.8.2. Exercise progression (EX; control)
Exercise training sessions consist of resistance-based exercises performed in a group setting dedicated to the study at a local facility (CrossFit Bloomington). All training sessions are led and supervised by a certified coach who adapts the workouts based on individual needs and ability. Each 1 h session consists of a 20 min warm-up that includes instruction and demonstration of the workout, dynamic mobility drills involving the major muscle groups and joints used in the main exercise session; a 20 min resistance-based circuit training workout consisting of multiple, self-paced sets of 4–6 exercises; and a 20 min cool-down involving 5 min of aerobic movement (e.g., stationary cycling) and static stretching of the major muscle groups used during the main exercise session. Minimal rest (<30 s) will be encouraged during the transition between movements and sets. Exercise session intensity will be monitored continuously by participants and supervising coaches/research team members using a Polar heart rate monitor (H10, Polar Electro, Kempele, Finland) connected via Bluetooth to the Polar Beat phone application. Session intensity will be adjusted (e.g., to maintain a prescribed target heart rate that will progress gradually from 40 to 50% of heart rate reserve (HRR) at week 1 to 60–70% HRR at week 8. Additional details of the exercise training program are provided in Table 2.
Table 2.
Details of the 8 week resistance-based circuit training program.
| Weeks | Day | Exercises | Duration | Prescribed Intensity |
|---|---|---|---|---|
| All | All | Warm-up: dynamic mobility drills for the major muscle groups and joints used during the main exercise session, instruction and demonstration of main exercises | 20 min | |
| 1, 3, 5, 7 | 1 | 5 sets of: 1:00 - weighted farmer's carry 1:00 - AbMat sit-up 1:00 – weighted farmer's carry 1:00 – stationary cycling |
20 min | Week 1: 40–50% HRR Week 3: 50–60% HRR Week 5: 60–70% HRR Week 7: 60–70% HRR |
| 2 | 15-12-9 repetitions of: Bodyweight squat Push-up/modified push-up Bodyweight ring row Stationary cycling for 15 Kcal, 12 Kcal, 9 Kcal |
20 min | Week 1: 40–50% HRR Week 3: 50–60% HRR Week 5: 50–60% HRR Week 7: 60–70% HRR |
|
| 3 | Assessment exercise: weighted farmer's carry Sets of: Stationary cycling for 15 Kcal 30 bodyweight squats 20 AbMat sit-ups 10 ring rows 5 push-ups/modified push-ups |
2 min 20 min |
Week 1: 40–50% HRR Week 3: 50–60% HRR Week 5: 50–60% HRR Week 7: 60–70% HRR |
|
| 2, 4, 6, 8 | 1 | 5 sets of: 1:00 – weighted wall-ball throws 1:00 – walking lunges 1:00 – weighted wall-ball throws 1:00 – stationary cycling |
20 min | Week 2: 40–50% HRR Week 4: 50–60% HRR Week 6: 50–60% HRR Week 8: 60–70% HRR |
| 2 | 15-12-9 repetitions of: Box step-up Modified burpee Push-up/modified push-up Stationary cycling 15 Kcal, 12 Kcal, 9 Kcal |
20 min | Week 2: 40–50% HRR Week 4: 50–60% HRR Week 6: 50–60% HRR Week 8: 60–70% HRR |
|
| 3 | Sets of: 10 modified burpees 10 push-ups/modified push-ups 20 walking lunges 15 AbMat sit-ups 20 weighted wall-ball throws |
20 min | Week 2: 40–50% HRR Week 4: 50–60% HRR Week 6: 50–60% HRR Week 8: 60–70% HRR |
|
| All | All | Cool-down: 5 min of aerobic activity, static stretching of major muscle groups used during the main exercise session | 20 min |
HRR = heart rate reserve.
3.8.3. Exercise progression with prior placebo consumption (EX + PL; placebo)
Participants randomized to the EX + PL group will be supplied with a placebo version of the 140 mL commercially-available beetroot juice (James White Drinks, UK) deplete of nitrate. The manufacturer extracts nitrate from the beetroot juice using an ion-exchange resin, which maintains the taste and appearance of the beverage.
The EX + PL and EX + BR groups will be initially supplied with a 4 week ration (24 bottles) of the beetroot or placebo beverages, which will then be repeated at the half-point of the exercise training progression. Participants will be instructed to consume the beverage between 2 and 3 h prior to each scheduled exercise training session (maximum 3 times per week) to ensure that the timing of the training session coincides with peak nitrate/nitrite bioavailability [36].
3.8.4. Exercise progression with prior beetroot consumption (EX + BR; experimental)
Participants randomized to the EX + BR experimental group will be supplied with 140 mL of commercially-available beetroot juice (James White Drinks, UK) containing approximately 12 mmol of nitrate. During the final training week, participants in the experimental group will be administered the placebo to negate any carry-over effects that could influence the follow-up assessment outcomes.
3.9. Quality control measures
The success of our exercise training/beetroot supplementation intervention is predicated on the assumption that participants are consuming an effective dose of dietary nitrate and are achieving an intensity during exercise sessions that is conducive for adaptations to occur. Accordingly, measures are taken to control the quality of the beetroot juice supplement and exercise session stimulus.
Due to the known variability in nitrate content of commercially-available beetroot juice, the supplement chosen (Beet It Sport Nitrate 400 Shot, James White Drinks, UK) has consistently provided nitrate content within manufacturer specifications [56]. The nitrate content of the beetroot juice and placebo beverages are additionally measured at random in accordance with previous work [56] to confirm product integrity.
While participants are instructed to maintain their usual diet during the intervention, to refrain from eating foods with a high nitrate content (e.g., leafy greens, beets) within 12 h of scheduled assessment visits, or from using antibacterial mouthwash (e.g., Listerine, Cepacol) for the entire study duration, they are not adhering to a controlled diet. The amount of nitrate consumed through usual diet will be estimated using a three-day dietary recall (i.e., two weekdays and one weekend day) obtained at weeks 1 and 8 of the intervention.
The progression and intensity of exercise training (from weeks 1–8) is monitored via continuous heart rate monitoring along with assessing post-exercise session rating of perceived exertion with the validated Borg CR10 scale [47]. At least three individuals affiliated with the study will be present at each training session to monitor exercise heart rate and make adjustments to encourage the participant to stay within the target heart rate zone (i.e., to increase their cadence while stationary cycling, select a heavier weight for farmer's carry). Participants will also be supplied with access to an online training journal, in which they will record the total amount of repetitions and sets of exercises performed during each session. Research team members will be given remote access to the online training journal and periodic checks will be made to assess compliance.
The first week of exercise training consists of two instructional sessions wherein participants will become familiarized with the workout structure, equipment, basic movements, and potential exercise modifications. On the third session of the first week, participants will complete a series of baseline tests including consisting of a loaded carry (i.e., weighted farmer's carry) with a self-selected weight for 2 min followed by sets of resistance exercises (e.g., bodyweight squats, sit-ups, ring rows, push-ups/modified push-ups). The amount of mechanical work completed for the farmer's walk is calculated as distance traveled (m) x weight (kg) in 2 min and normalized to body mass. Additionally, the number of sets performed for the specified resistance exercises are quantified. Exercise testing will be completed again during the 3rd, 5th, and 7th week of intervention to evaluate changes in mechanical work during the farmer's carry and performance on the designated resistance exercises.
Participants are withdrawn from study participation if they miss more than three consecutive exercise sessions in any given week across the 8 week training intervention. Additionally, they must complete a minimum of 70% of the total scheduled exercise training sessions (17 out of 24 sessions attended) to continue with follow-up assessments.
A data safety monitor, independent of the primary investigator and funding source, has been appointed to oversee aspects of data management/security and safety monitoring. Periodic inspections of data quality and random assessments of participant recruitment, retention, adherence, and adverse events will be performed. In addition, formal meetings between the study site coordinator (M.N.B) and the data safety monitor will occur every 3 months to address items related to data quality.
3.10. Participant safety
Precautionary measures are followed to encourage participant safety with all study activities. Participants are required to obtain physician clearance prior to enrollment, and additionally, screened by research personnel for past history, signs, and symptoms of cardiac, pulmonary, or metabolic disease that would preclude safe participation in exercise as determined by a medical health history questionnaire consistent with the American College of Sports Medicine guidelines [57].
The potential for unintended/adverse effects related to acute beetroot juice ingestion are monitored during the first baseline assessment visit. Participants ingest 140 mL of the commercially-available beetroot juice in a controlled laboratory setting while measures of blood pressure and methemoglobin saturation (SpMet®, Masimo, CH) are taken each hour for three consecutive hours. Consistent with previous work [58], a reduction of ≥20 mm Hg in systolic blood pressure or value ≥ 12% in blood methemoglobin saturation measured using a CO-oximeter is indicative of an adverse event precluding further participation [59]. In agreement with local institutional policy, all adverse events are reported to the Institutional Review Board.
3.11. Sample size and data analysis considerations
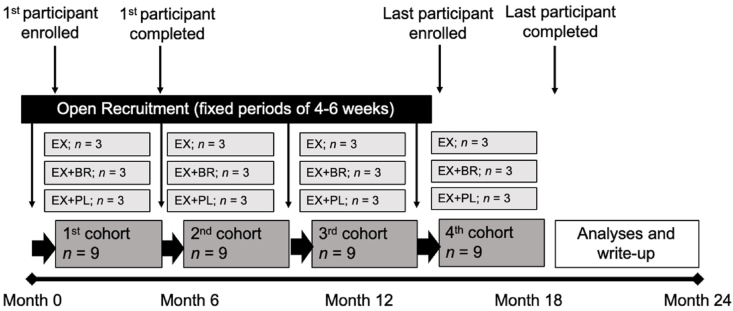
This project primarily seeks to determine elements of feasibility and preliminary effect sizes, such that our data will inform development of a larger-scale clinical trial to confirm efficacy. Due to the novelty of exercise training coupled with beetroot juice consumption, our group was unable to perform a priori power analyses for sample size estimates. However, previous accounts have observed large beneficial effects on mean arterial blood pressure, pulse wave velocity, flow-mediated vasodilation, plasma nitrite/nitrate concentrations, and 6 min walk distance following combined resistance and aerobic exercise training protocols [60,61] and chronic dietary nitrate interventions [20] in samples of 10–12 postmenopausal women per group. In addition, a sample size of 12 per group has been recommended for parallel design pilot studies to achieve a level of precision around mean and variance estimates used for future sample size calculations [62]. Fig. 2 illustrates a schematic timeline for anticipated enrollment.
Fig. 2.
Recruitment and enrollment of participants will occur in fixed periods of 4–6 weeks over the course of approximately 18 months. The minimum duration for each cohort from enrollment to the final follow-up visit is 10 weeks. EX, exercise training only. EX + PL, exercise training with placebo. EX + BR, exercise training with beetroot juice.
Descriptive statistics and calculations of rates (e.g., recruitment, retention, adherence, and adverse events) will be conducted to fulfill the primary study aim. Outcomes for secondary and exploratory aims will be analyzed using mixed models and analyses of covariance (ANCOVA). To compare the effects of EX + BR and EX + PL against EX, only data from subjects who complete at least 70% of the total possible exercise training sessions (17 out of 24 sessions) will be included in the final analyses.
4. Discussion
Postmenopausal women report higher rates of disability and CVD compared with men of a similar age [2,3]. Age-related oxidative stress is a likely factor contributing to the loss in nitric oxide and attendant decline in physiologic function during and after the menopausal transition [8,9]. The BEE SWEET trial is designed to evaluate the feasibility of dietary nitrate, consumed 2–3 h prior to exercise, as a means to promote adaptive responses to habitual training programs. Preliminary effect sizes will be used to direct future randomized controlled trials intended to confirm the translational application of dietary nitrate coupled with exercise training to reduce oxidative stress and inflammation following menopause.
Exercise training is a highly effective, evidence-based approach to restore vascular function with aging [63,64]. In previously sedentary older men (aged 55–79 years), 8 weeks of brisk walking elicited a >50% increase in brachial artery endothelium-dependent vasodilation as assessed by flow-mediated dilation [7]. However, the same exercise prescription had no impact on the large-artery function of age-matched women [7]. Further data support this finding, suggesting the responsiveness to habitual exercise training may be sex- and mode-specific in older adults, with postmenopausal women inconsistently benefiting from habitual aerobic exercise training programs [6,63]. Of note, resistance [[65], [66], [67]] and combined aerobic/resistance exercise training protocols [60,61] have reduced systolic and diastolic blood pressure in postmenopausal women conceivably due to alterations in autonomic balance that may be dysregulated following menopause [4,68]. Autonomic dysfunction has previously been observed in 67% of generally healthy postmenopausal women aged 55–65 years [68]. Sex-specific differences conceivably exist due to additional estrogen-related impairments in nitric oxide signaling among older women [69]. Accordingly, restoration of estrogen through acute hormone replacement treatment has resulted in improvements in vascular function following aerobic exercise training interventions in estrogen-deficient postmenopausal women [69]. The use of estrogen-replacement therapy is controversial, however, and has not proven to be an effective long-term strategy for reducing CVD risk, particularly for women in late menopause [15,16]. The present trial was developed to evaluate the potential utility of dietary nitrate (beetroot juice) as an alternative strategy to restore the loss in nitric oxide signal transduction and enhance vascular responsiveness with habitual resistance exercise training in a cohort of postmenopausal women.
Indeed, the BEE SWEET trial seeks to evaluate preliminary effect sizes of pre-exercise dietary nitrate (beetroot juice) on changes in endothelial-dependent vasodilation following habitual exercise training. From a mechanistic perspective, we reason that the additive effects of nitric oxide - derived through chronic beetroot juice ingestion and repetitive shear stress invoked during resistance exercise training sessions (3 times per week for 8 weeks) [70] - will result in more robust endothelium-dependent vascular adaptations when compared to exercise training alone. Separately, dietary nitrate and habitual exercise training interventions have favorably influenced blood pressure [19,[71], [72], [73]], macrovascular [7,73,74], and microvascular function [75] in healthy older men and women. However, the cumulative effect of dietary nitrate coupled with exercise training has not yet been compared to exercise training alone, and it is further unknown whether such interventions will elicit differing adaptations in conduit or resistance vessels [63,73]. As such, we are assessing changes in both macrovascular (brachial artery diameter) and microvasculature (cutaneous vascular conductance) function using flow-mediated vasodilation and laser-Doppler flowmetry, respectively, following EX + BR compared with EX and EX + PL.
Additional evidence supports the premise of our hypothesis. Specifically, pre-exercise dietary nitrate will augment the intensity of exercise training sessions. Owing to reduced oxygen cost and/or improvement in muscle contractile efficiency [76,77], dietary nitrate supplementation has been shown to result in a higher self-selected intensity [78], extended time to exhaustion [79,80] and lower perceptions of effort during exercise [79] in younger adults. An improved ability to tolerate a single bout of exercise could lead to more potent exercise training-effects by allowing participants to exercise at a greater intensity each session. However, despite evidence that dietary nitrate supplementation improves submaximal exercise tolerance in older adults with underlying chronic disease [81,82], studies evaluating the effectiveness of dietary nitrate on exercise performance in healthy older adults is limited and conflicting [83]. As such, we are monitoring evidence of improved exercise tolerance through ratings of perceived session difficulty (Borg CR10), session intensity (continuous heart rate) and volume (repetitions, sets, self-selected weight) across the exercise training intervention. Potential mechanisms of improved exercise tolerance, such as a reduced oxygen cost of walking and improved muscle contractile properties are also evaluated, as well as outcomes of functional mobility that have clinical implications including the 6 min walk test and walking self-efficacy.
While this preliminary approach is designed to evaluate feasibility and preliminary effect sizes for acute changes in endothelial-dependent vasodilation and functional mobility following an 8 week exercise training period, the maintenance of behavior-modifications following health-related interventions is key in evaluating their success [84,85]. As such, to fully understand the translational application of dietary nitrate coupled with exercise training on health outcomes in postmenopausal women, additional longitudinal trials with sustained follow-up periods are necessary. Importantly, long-term alterations in free-living physical activity habits evaluated by accelerometry or doubly-labelled water are strongly associated with risk of developing CVD [86,87], functional disability [88] and all-cause mortality [89]. The exit-evaluation administered at the conclusion of the BEE SWEET trial is meant to guide future studies that will investigate the influence of dietary nitrate supplementation on behavior modifications following the intervention period. Participants will be asked whether they think they consumed beetroot juice or placebo and whether this affected their ability to exercise. Additionally, participants will be asked to indicate their likelihood of continuing with an exercise program. Findings from the BEE SWEET trial will direct further work aimed at tracking changes in free-living physical activity and health behaviors associated with mobility and CVD risk following similar EX = BR interventions.
5. Conclusions
In conclusion, the BEE SWEET trial may reveal that coupling pre-exercise dietary nitrate with structured exercise training is a feasible approach to improve functional mobility and endothelial-dependent vasodilation in postmenopausal women. Results will inform the design of a larger-scale, randomized controlled trial to evaluate efficacy of dietary nitrate with exercise training to elicit longer-term behavioral modifications that attenuate CVD risk in postmenopausal women.
Clinical trials identifier
Funding
This work was supported by the Indiana Clinical and Translational Sciences Institute funded by grant number UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award, and by grant number AG053606 from the National Institute on Aging.
Declaration of competing interest
The authors have no conflicts of interest to declare.
References
- 1.Ortman J.M., Velkoff V.A., Hogan H. An Aging Nation: the Older Population in the United States. In: Do Commerce., editor. 2014. [Google Scholar]
- 2.Leveille S.G., Resnick H.E., Balfour J. Gender differences in disability: evidence and underlying reasons. Aging (Milano) 2000;12(2):106–112. doi: 10.1007/bf03339897. Epub 2000/07/21. PubMed PMID: 10902052. [DOI] [PubMed] [Google Scholar]
- 3.Mosca L., Barrett-Connor E., Wenger N.K. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124(19):2145–2154. doi: 10.1161/circulationaha.110.968792. Epub 2011/11/09. PubMed PMID: 22064958; PubMed Central PMCID: PMCPMC3362050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker B.A., Kalasky M.J., Proctor D.N. Evidence for sex differences in cardiovascular aging and adaptive responses to physical activity. Eur. J. Appl. Physiol. 2010;110(2):235–246. doi: 10.1007/s00421-010-1506-7. Epub 2010/05/18. PubMed PMID: 20480371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore D.J., Proctor D.N. Found in ‘transition’: shifting mechanisms of aerobic exercise adaptation in ageing women. J. Physiol. 2017;595(13):4119–4120. doi: 10.1113/jp274163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreau K.L., Ozemek C. Vascular adaptations to habitual exercise in older adults: time for the sex talk. Exerc. Sport Sci. Rev. 2017;45(2):116–123. doi: 10.1249/jes.0000000000000104. Epub 2017/01/17. PubMed PMID: 28092297; PubMed Central PMCID: PMCPMC5357172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierce G.L., Eskurza I., Walker A.E., Fay T.N., Seals D.R. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin. Sci. (Lond.) 2011;120(1):13–23. doi: 10.1042/cs20100174. Epub 2010/07/21. PubMed PMID: 20642454; PubMed Central PMCID: PMCPMC3809822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreau K.L., Hildreth K.L., Meditz A.L., Deane K.D., Kohrt W.M. Endothelial function is impaired across the stages of the menopause transition in healthy women. J. Clin. Endocrinol. Metab. 2012;97(12):4692–4700. doi: 10.1210/jc.2012-2244. Epub 2012/09/13. PubMed PMID: 22969140; PubMed Central PMCID: PMCPMC3513538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taddei S., Virdis A., Ghiadoni L., Salvetti G., Bernini G., Magagna A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38(2):274–279. doi: 10.1161/01.hyp.38.2.274. Epub 2001/08/18. PubMed PMID: 11509489. [DOI] [PubMed] [Google Scholar]
- 10.Hobbs D.A., George T.W., Lovegrove J.A. The effects of dietary nitrate on blood pressure and endothelial function: a review of human intervention studies. Nutr. Res. Rev. 2013;26(2):210–222. doi: 10.1017/s0954422413000188. Epub 2013/10/19. PubMed PMID: 24134873. [DOI] [PubMed] [Google Scholar]
- 11.Coggan A.R., Peterson L.R. Dietary nitrate enhances the contractile properties of human skeletal muscle. Exerc. Sport Sci. Rev. 2018;46(4):254–261. doi: 10.1249/jes.0000000000000167. Epub 2018/07/13. PubMed PMID: 30001275; PubMed Central PMCID: PMCPMC6138552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tousoulis D., Kampoli A.M., Tentolouris C., Papageorgiou N., Stefanadis C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012;10(1):4–18. doi: 10.2174/157016112798829760. Epub 2011/11/25. PubMed PMID: 22112350. [DOI] [PubMed] [Google Scholar]
- 13.Kingwell B.A. Nitric oxide as a metabolic regulator during exercise: effects of training in health and disease. Clin. Exp. Pharmacol. Physiol. 2000;27(4):239–250. doi: 10.1046/j.1440-1681.2000.03232.x. Epub 2000/04/25. PubMed PMID: 10779120. [DOI] [PubMed] [Google Scholar]
- 14.Nevzati E., Shafighi M., Bakhtian K.D., Treiber H., Fandino J., Fathi A.R. Estrogen induces nitric oxide production via nitric oxide synthase activation in endothelial cells. Acta Neurochir. Suppl. 2015;120:141–145. doi: 10.1007/978-3-319-04981-6_24. Epub 2014/11/05. PubMed PMID: 25366614. [DOI] [PubMed] [Google Scholar]
- 15.Iorga A., Cunningham C.M., Moazeni S., Ruffenach G., Umar S., Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017;8(1):33. doi: 10.1186/s13293-017-0152-8. Epub 2017/10/27. PubMed PMID: 29065927; PubMed Central PMCID: PMCPMC5655818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossouw J.E., Manson J.E., Kaunitz A.M., Anderson G.L. Lessons learned from the Women's Health Initiative trials of menopausal hormone therapy. Obstet. Gynecol. 2013;121(1):172–176. doi: 10.1097/aog.0b013e31827a08c8. http://10.1097/AOG.0b013e31827a08c8.10.1097/aog.0b013e31827a08c8 Epub 2012/12/25. PubMed PMID: 23262943; PubMed Central PMCID: PMCPMC3547645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doel J.J., Benjamin N., Hector M.P., Rogers M., Allaker R.P. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur. J. Oral Sci. 2005;113(1):14–19. doi: 10.1111/j.1600-0722.2004.00184.x. Epub 2005/02/08. PubMed PMID: 15693824. [DOI] [PubMed] [Google Scholar]
- 18.Kapil V., Khambata R.S., Robertson A., Caulfield M.J., Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65(2):320–327. doi: 10.1161/hypertensionaha.114.04675. Epub 2014/11/26. PubMed PMID: 25421976; PubMed Central PMCID: PMCPMC4288952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim D.J., Roe C.A., Somani Y.B., Moore D.J., Barrett M.A., Flanagan M. Effects of acute dietary nitrate supplementation on aortic blood pressures and pulse wave characteristics in post-menopausal women. Nitric Oxide. 2019;85:10–16. doi: 10.1016/j.niox.2019.01.008. Epub 2019/01/23. PubMed PMID: 30668996; PubMed Central PMCID: PMCPMC6389403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayra S.T., Johnston C.S., Sweazea K.L. High-nitrate salad increased plasma nitrates/nitrites and brachial artery flow-mediated dilation in postmenopausal women: a pilot study. Nutr. Res. 2019;65:99–104. doi: 10.1016/j.nutres.2019.02.001. Epub 2019/04/08. PubMed PMID: 30954341. [DOI] [PubMed] [Google Scholar]
- 21.Bailey S.J., Varnham R.L., DiMenna F.J., Breese B.C., Wylie L.J., Jones A.M. Inorganic nitrate supplementation improves muscle oxygenation, O(2) uptake kinetics, and exercise tolerance at high but not low pedal rates. J. Appl. Physiol. 1985;118(11):1396–1405. doi: 10.1152/japplphysiol.01141.2014. Epub 2015/04/11. PubMed PMID: 25858494. [DOI] [PubMed] [Google Scholar]
- 22.Jajja A., Sutyarjoko A., Lara J., Rennie K., Brandt K., Qadir O. Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr. Res. 2014;34(10):868–875. doi: 10.1016/j.nutres.2014.09.007. Epub 2014/10/09. PubMed PMID: 25294299. [DOI] [PubMed] [Google Scholar]
- 23.Jones A.M. Dietary nitrate supplementation and exercise performance. Sports Med. 2014;44(Suppl 1):S35–S45. doi: 10.1007/s40279-014-0149-y. Epub 2014/05/06. PubMed PMID: 24791915; PubMed Central PMCID: PMCPMC4008816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker M.A., Bailey T.G., McIlvenna L., Allen J.D., Green D.J., Askew C.D. Acute dietary nitrate supplementation improves flow mediated dilatation of the superficial femoral artery in healthy older males. Nutrients. 2019;11(5) doi: 10.3390/nu11050954. Epub 2019/05/01. PubMed PMID: 31035478; PubMed Central PMCID: PMCPMC6566150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. PubMed PMID: 24324449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connelly L., Palacios-Callender M., Ameixa C., Moncada S., Hobbs A.J. Biphasic regulation of NF-kappa B activity underlies the pro- and anti-inflammatory actions of nitric oxide. J. Immunol. 2001;166(6):3873–3881. doi: 10.4049/jimmunol.166.6.3873. Epub 2001/03/10. PubMed PMID: 11238631. [DOI] [PubMed] [Google Scholar]
- 27.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029. Epub 2007/01/24. 2006. PubMed PMID: 17237348; PubMed Central PMCID: PMCPMC2248324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundberg J.O., Carlstrom M., Larsen F.J., Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc. Res. 2011;89(3):525–532. doi: 10.1093/cvr/cvq325. Epub 2010/10/13. PubMed PMID: 20937740. [DOI] [PubMed] [Google Scholar]
- 29.Sindler A.L., Devan A.E., Fleenor B.S., Seals D.R. Inorganic nitrite supplementation for healthy arterial aging. J. Appl. Physiol. 1985;116(5):463–477. doi: 10.1152/japplphysiol.01100. Epub 2014/01/11. 2013. PubMed PMID: 24408999; PubMed Central PMCID: PMCPMC3949212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenjale A.A., Ham K.L., Stabler T., Robbins J.L., Johnson J.L., Vanbruggen M. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J. Appl. Physiol. 1985;110(6):1582–1591. doi: 10.1152/japplphysiol.00071. Epub 2011/04/02. 2011. PubMed PMID: 21454745; PubMed Central PMCID: PMCPMC3119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruse N.T., Ueda K., Hughes W.E., Casey D.P. Eight weeks of nitrate supplementation improves blood flow and reduces the exaggerated pressor response during forearm exercise in peripheral artery disease. Am. J. Physiol. Heart Circ. Physiol. 2018;315(1) doi: 10.1152/ajpheart.00015. H101-h8. Epub 2018/03/10. 2018. PubMed PMID: 29522355; PubMed Central PMCID: PMCPMC6087779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh S.M., Kapil V., Fuentes-Calvo I., Bubb K.J., Pearl V., Milsom A.B. Enhanced vasodilator activity of nitrite in hypertension: critical role for erythrocytic xanthine oxidoreductase and translational potential. Hypertension. 2013;61(5):1091–1102. doi: 10.1161/hypertensionaha.111.00933. Epub 2013/04/17. PubMed PMID: 23589565. [DOI] [PubMed] [Google Scholar]
- 33.de Oliveira G.V., Morgado M., Conte-Junior C.A., Alvares T.S. Acute effect of dietary nitrate on forearm muscle oxygenation, blood volume and strength in older adults: a randomized clinical trial. PloS One. 2017;12(11) doi: 10.1371/journal.pone.0188893. e0188893. Epub 2017/12/01. PubMed PMID: 29190751; PubMed Central PMCID: PMCPMC5708833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coggan A.R., Leibowitz J.L., Kadkhodayan A., Thomas D.P., Ramamurthy S., Spearie C.A. Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric Oxide. 2015;48:16–21. doi: 10.1016/j.niox.2014.08.014. Epub 2014/09/10. PubMed PMID: 25199856; PubMed Central PMCID: PMCPMC4362985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coggan A.R., Hoffman R.L., Gray D.A., Moorthi R.N., Thomas D.P., Leibowitz J.L. A single dose of dietary nitrate increases maximal knee extensor angular velocity and power in healthy older men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75(6):1154–1160. doi: 10.1093/gerona/glz156. Epub 2019/06/25. PubMed PMID: 31231758; PubMed Central PMCID: PMCPMC7243590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webb A.J., Patel N., Loukogeorgakis S., Okorie M., Aboud Z., Misra S. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51(3):784–790. doi: 10.1161/hypertensionaha.107.103523. Epub 2008/02/06. PubMed PMID: 18250365; PubMed Central PMCID: PMCPMC2839282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Modin A., Bjorne H., Herulf M., Alving K., Weitzberg E., Lundberg J.O. Nitrite-derived nitric oxide: a possible mediator of 'acidic-metabolic' vasodilation. Acta Physiol. Scand. 2001;171(1):9–16. doi: 10.1046/j.1365-201X.2001.00771.x. Epub 2001/05/15. PubMed PMID: 11350258. [DOI] [PubMed] [Google Scholar]
- 38.Richardson R.S., Noyszewski E.A., Kendrick K.F., Leigh J.S., Wagner P.D. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J. Clin. Invest. 1995;96(4):1916–1926. doi: 10.1172/jci118237. Epub 1995/10/01. PubMed PMID: 7560083; PubMed Central PMCID: PMCPMC185828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Circular Area profiles (CAPS) - 2010. http://mcdc.missouri.edu/applications/caps2010.html Missouri Census Data Center; [updated April 19, 2017; cited 2020 August 31]. Available from:
- 40.ATS statement: guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. Epub 2002/07/02. PubMed PMID: 12091180. [DOI] [PubMed] [Google Scholar]
- 41.Costa M., Goldberger A.L., Peng C.K. Multiscale entropy analysis of biological signals. Phys. Rev. E - Stat. Nonlinear Soft Matter Phys. 2005;71(2 Pt 1) doi: 10.1103/PhysRevE.71.021906. 021906. Epub 2005/03/24. PubMed PMID: 15783351. [DOI] [PubMed] [Google Scholar]
- 42.Harada N.D., Chiu V., Stewart A.L. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch. Phys. Med. Rehabil. 1999;80(7):837–841. doi: 10.1016/s0003-9993(99)90236-8. Epub 1999/07/22. PubMed PMID: 10414771. [DOI] [PubMed] [Google Scholar]
- 43.Rikli R.E., Jones C.J. The reliability and validity of a 6-minute walk test as a measure of physical endurance in older adults. 1998;6(4):363. doi: 10.1123/japa.6.4.363. [DOI] [Google Scholar]
- 44.Tomás M.T., Galán-Mercant A., Carnero E.A., Fernandes B. Functional capacity and levels of physical activity in aging: a 3-year follow-up. Front. Med. 2018;4:244. doi: 10.3389/fmed.2017.00244. PubMed PMID: 29376052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kosse N.M., Vuillerme N., Hortobágyi T., Lamoth C.J. Multiple gait parameters derived from iPod accelerometry predict age-related gait changes. Gait Posture. 2016;46:112–117. doi: 10.1016/j.gaitpost.2016.02.022. Epub 2016/05/01. PubMed PMID: 27131187. [DOI] [PubMed] [Google Scholar]
- 46.Bisi M.C., Stagni R. Complexity of human gait pattern at different ages assessed using multiscale entropy: from development to decline. Gait Posture. 2016;47:37–42. doi: 10.1016/j.gaitpost.2016.04.001. Epub 2016/06/07. PubMed PMID: 27264400. [DOI] [PubMed] [Google Scholar]
- 47.Borg G. 1998. Borg's Perceived Exertion and Pain Scales Human Kinetics. [Google Scholar]
- 48.McAuley E., Blissmer B., Katula J., Duncan T.E. Exercise environment, self-efficacy, and affective responses to acute exercise in older adults. Psychol. Health. 2000;15:341–357. [Google Scholar]
- 49.Coggan A.R., Broadstreet S.R., Mikhalkova D., Bole I., Leibowitz J.L., Kadkhodayan A. Dietary nitrate-induced increases in human muscle power: high versus low responders. Physiol. Rep. 2018;6(2) doi: 10.14814/phy2.13575. Epub 2018/01/26. PubMed PMID: 29368802; PubMed Central PMCID: PMCPMC5789728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dubé M.P., Shen C., Mather K.J., Waltz J., Greenwald M., Gupta S.K. Relationship of body composition, metabolic status, antiretroviral use, and HIV disease factors to endothelial dysfunction in HIV-infected subjects. AIDS Res. Hum. Retrovir. 2010;26(8):847–854. doi: 10.1089/aid.2010.0007. PubMed PMID: 20673142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta S.K., Mi D., Moe S.M., Dubé M.P., Liu Z. Effects of switching from efavirenz to raltegravir on endothelial function, bone mineral metabolism, inflammation, and renal function: a randomized, controlled trial. J. Acquir. Immune Defic. Syndr. 2013;64(3):279–283. doi: 10.1097/qai.0b013e3182a97c39. PubMed PMID: 24278992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi P.J., Brunt V.E., Fujii N., Minson C.T. New approach to measure cutaneous microvascular function: an improved test of NO-mediated vasodilation by thermal hyperemia. J. Appl. Physiol. 1985;117(3):277–283. doi: 10.1152/japplphysiol.01397.2013. Epub 2014/06/07. PubMed PMID: 24903917; PubMed Central PMCID: PMCPMC4122693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wylie L.J., Park J.W., Vanhatalo A., Kadach S., Black M.I., Stoyanov Z. Human skeletal muscle nitrate store: influence of dietary nitrate supplementation and exercise. J. Physiol. 2019;597(23):5565–5576. doi: 10.1113/jp278076. Epub 2019/07/28. PubMed PMID: 31350908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkerson D.P., Hayward G.M., Bailey S.J., Vanhatalo A., Blackwell J.R., Jones A.M. Influence of acute dietary nitrate supplementation on 50 mile time trial performance in well-trained cyclists. Eur. J. Appl. Physiol. 2012;112(12):4127–4134. doi: 10.1007/s00421-012-2397-6. Epub 2012/04/25. PubMed PMID: 22526247. [DOI] [PubMed] [Google Scholar]
- 55.Marteus H., Tornberg D.C., Weitzberg E., Schedin U., Alving K. Origin of nitrite and nitrate in nasal and exhaled breath condensate and relation to nitric oxide formation. Thorax. 2005;60(3):219–225. doi: 10.1136/thx.2004.030635. Epub 2005/03/03. PubMed PMID: 15741439; PubMed Central PMCID: PMCPMC1747344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gallardo E.J., Coggan A.R. What's in your beet juice? Nitrate and nitrite content of beet juice products marketed to athletes. Int. J. Sport Nutr. Exerc. Metabol. 2019;29(4):345–349. doi: 10.1123/ijsnem.2018-0223. Epub 2018/10/10. PubMed PMID: 30299195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Medicine ACoS . 10 ed. Wolters Kluwer Health; Philadelphia: 2018. ACSM's Guidelines for Exercise Testing and Prescription. [Google Scholar]
- 58.DeVan A.E., Johnson L.C., Brooks F.A., Evans T.D., Justice J.N., Cruickshank-Quinn C. Effects of sodium nitrite supplementation on vascular function and related small metabolite signatures in middle-aged and older adults. J. Appl. Physiol. 1985;120(4):416–425. doi: 10.1152/japplphysiol.00879. Epub 2015/11/27. PubMed PMID: 26607249; PubMed Central PMCID: PMCPMC4754621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skold A., Cosco D.L., Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South. Med. J. 2011;104(11):757–761. doi: 10.1097/SMJ.0b013e318232139f. Epub 2011/10/26. PubMed PMID: 22024786. [DOI] [PubMed] [Google Scholar]
- 60.Son W.M., Sung K.D., Cho J.M., Park S.Y. Combined exercise reduces arterial stiffness, blood pressure, and blood markers for cardiovascular risk in postmenopausal women with hypertension. Menopause. 2017;24(3):262–268. doi: 10.1097/gme.0000000000000765. Epub 2016/10/26. PubMed PMID: 27779565. [DOI] [PubMed] [Google Scholar]
- 61.Figueroa A., Park S.Y., Seo D.Y., Sanchez-Gonzalez M.A., Baek Y.H. Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause. 2011;18(9):980–984. doi: 10.1097/gme.0b013e3182135442. Epub 2011/05/05. PubMed PMID: 21540753. [DOI] [PubMed] [Google Scholar]
- 62.Julious S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut. Stat. 2005;4(4):287–291. doi: 10.1002/pst.185. [DOI] [Google Scholar]
- 63.Seals D.R., Nagy E.E., Moreau K.L. Aerobic exercise training and vascular function with ageing in healthy men and women. J. Physiol. 2019;597(19):4901–4914. doi: 10.1113/jp277764. Epub 2019/05/12. PubMed PMID: 31077372; PubMed Central PMCID: PMCPMC6773490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seals D.R., Walker A.E., Pierce G.L., Lesniewski L.A. Habitual exercise and vascular ageing. J. Physiol. 2009;587(Pt 23):5541–5549. doi: 10.1113/jphysiol.2009.178822. Epub 2009/09/03. PubMed PMID: 19723776; PubMed Central PMCID: PMCPMC2805366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gelecek N., İlçin N., Subaşi S.S., Acar S., Demir N., Örmen M. The effects of resistance training on cardiovascular disease risk factors in postmenopausal women: a randomized-controlled trial. Health Care Women Int. 2012;33(12):1072–1085. doi: 10.1080/07399332.2011.645960. [DOI] [PubMed] [Google Scholar]
- 66.Shaw B.S., Gouveia M., McIntyre S., Shaw I. Anthropometric and cardiovascular responses to hypertrophic resistance training in postmenopausal women. Menopause. 2016;23(11):1176–1181. doi: 10.1097/gme.0000000000000687. Epub 2016/10/26. PubMed PMID: 27433861. [DOI] [PubMed] [Google Scholar]
- 67.El-Refaye G., Younis H. The effect of 12 weeks of resistive exercises versus aerobic exercises in overweight hypertensive postmenopausal women. Bull. Faculty Phys. Ther. 2019;24(1):40–48. doi: 10.4103/bfpt.bfpt_19_18. [DOI] [Google Scholar]
- 68.Yalamudi K. Study of comparison between autonomic dysfunction and dyslipidemia in healthy postmenopausal women. J. Midlife Health. 2017;8(3):103–109. doi: 10.4103/jmh.JMH_67_15. PubMed PMID: 28983156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moreau K.L., Stauffer B.L., Kohrt W.M., Seals D.R. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J. Clin. Endocrinol. Metab. 2013;98(11):4507–4515. doi: 10.1210/jc.2013-2183. Epub 2013/10/05. PubMed PMID: 24092827; PubMed Central PMCID: PMCPMC3816259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Collier S.R., Kanaley J.A., Carhart R., Frechette V., Tobin M.M., Hall A.K. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J. Hum. Hypertens. 2008;22(10):678–686. doi: 10.1038/jhh.2008.36. [DOI] [PubMed] [Google Scholar]
- 71.Raubenheimer K., Hickey D., Leveritt M., Fassett R., Ortiz de Zevallos Munoz J., Allen J.D. Acute effects of nitrate-rich beetroot juice on blood pressure, hemostasis and vascular inflammation markers in healthy older adults: a randomized, placebo-controlled crossover study. Nutrients. 2017;9(11) doi: 10.3390/nu9111270. Epub 2017/11/23. PubMed PMID: 29165355; PubMed Central PMCID: PMCPMC5707742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stanaway L., Rutherfurd-Markwick K., Page R., Wong M., Jirangrat W., Teh K.H. Acute supplementation with nitrate-rich beetroot juice causes a greater increase in plasma nitrite and reduction in blood pressure of older compared to younger adults. Nutrients. 2019;11(7) doi: 10.3390/nu11071683. Epub 2019/07/25. PubMed PMID: 31336633; PubMed Central PMCID: PMCPMC6683255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones T., Dunn E.L., Macdonald J.H., Kubis H.P., McMahon N., Sandoo A. The effects of beetroot juice on blood pressure, microvascular function and large-vessel endothelial function: a randomized, double-blind, placebo-controlled pilot study in healthy older adults. Nutrients. 2019;11(8) doi: 10.3390/nu11081792. Epub 2019/08/07. PubMed PMID: 31382524; PubMed Central PMCID: PMCPMC6722817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Landers-Ramos R.Q., Corrigan K.J., Guth L.M., Altom C.N., Spangenburg E.E., Prior S.J. Short-term exercise training improves flow-mediated dilation and circulating angiogenic cell number in older sedentary adults. Appl. Physiol. Nutr. Metabol. 2016;41(8):832–841. doi: 10.1139/apnm-2015-0637. Epub 2016/07/22. PubMed PMID: 27441589; PubMed Central PMCID: PMCPMC5456122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hurley D.M., Williams E.R., Cross J.M., Riedinger B.R., Meyer R.A., Abela G.S. Aerobic exercise improves microvascular function in older adults. Med. Sci. Sports Exerc. 2019;51(4):773–781. doi: 10.1249/mss.0000000000001854. Epub 2018/11/30. PubMed PMID: 30489496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bailey S.J., Winyard P., Vanhatalo A., Blackwell J.R., Dimenna F.J., Wilkerson D.P. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 1985;107(4):1144–1155. doi: 10.1152/japplphysiol.00722.2009. Epub 2009/08/08. PubMed PMID: 19661447. [DOI] [PubMed] [Google Scholar]
- 77.Bailey S.J., Fulford J., Vanhatalo A., Winyard P.G., Blackwell J.R., DiMenna F.J. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol. 1985;109(1):135–148. doi: 10.1152/japplphysiol.00046.2010. Epub 2010/05/15. PubMed PMID: 20466802. [DOI] [PubMed] [Google Scholar]
- 78.Jo E., Fischer M., Auslander A.T., Beigarten A., Daggy B., Hansen K. The effects of multi-day vs. Single pre-exercise nitrate supplement dosing on simulated cycling time trial performance and skeletal muscle oxygenation. J. Strength Condit Res. 2019;33(1):217–224. doi: 10.1519/jsc.0000000000001958. Epub 2017/04/27. PubMed PMID: 28445231. [DOI] [PubMed] [Google Scholar]
- 79.Husmann F., Bruhn S., Mittlmeier T., Zschorlich V., Behrens M. Dietary nitrate supplementation improves exercise tolerance by reducing muscle fatigue and perceptual responses. Front. Physiol. 2019;10:404. doi: 10.3389/fphys.2019.00404. Epub 2019/05/10. PubMed PMID: 31068827; PubMed Central PMCID: PMCPMC6491676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lansley K.E., Winyard P.G., Fulford J., Vanhatalo A., Bailey S.J., Blackwell J.R. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J. Appl. Physiol. 1985;110(3):591–600. doi: 10.1152/japplphysiol.01070.2010. Epub 2010/11/13. PubMed PMID: 21071588. [DOI] [PubMed] [Google Scholar]
- 81.Berry M.J., Justus N.W., Hauser J.I., Case A.H., Helms C.C., Basu S. Dietary nitrate supplementation improves exercise performance and decreases blood pressure in COPD patients. Nitric Oxide. 2015;48:22–30. doi: 10.1016/j.niox.2014.10.007. Epub 2014/12/03. PubMed PMID: 25445634; PubMed Central PMCID: PMCPMC4411191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eggebeen J., Kim-Shapiro D.B., Haykowsky M., Morgan T.M., Basu S., Brubaker P. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2016;4(6):428–437. doi: 10.1016/j.jchf.2015.12.013. Epub 2016/02/15. PubMed PMID: 26874390; PubMed Central PMCID: PMCPMC4892939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stanaway L., Rutherfurd-Markwick K., Page R., Ali A. Performance and health benefits of dietary nitrate supplementation in older adults: a systematic review. Nutrients. 2017;9(11) doi: 10.3390/nu9111171. Epub 2017/10/28. PubMed PMID: 29077028; PubMed Central PMCID: PMCPMC5707643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ory M.G., Lee Smith M., Mier N., Wernicke M.M. The science of sustaining health behavior change: the health maintenance consortium. Am. J. Health Behav. 2010;34(6):647–659. doi: 10.5993/ajhb.34.6.2. Epub 2010/07/08. PubMed PMID: 20604691; PubMed Central PMCID: PMCPMC3753403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Henderson R.M., Miller M.E., Fielding R.A., Gill T.M., Glynn N.W., Guralnik J.M. Maintenance of physical function 1 Year after exercise intervention in at-risk older adults: follow-up from the LIFE study. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73(5):688–694. doi: 10.1093/gerona/glx231. Epub 2018/03/01. PubMed PMID: 29490012; PubMed Central PMCID: PMCPMC5905630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cochrane S.K., Chen S.H., Fitzgerald J.D., Dodson J.A., Fielding R.A., King A.C. Association of accelerometry-measured physical activity and cardiovascular events in mobility-limited older adults: the LIFE (lifestyle interventions and independence for elders) study. J. Am. Heart Assoc. 2017;6(12) doi: 10.1161/jaha.117.007215. Epub 2017/12/05. PubMed PMID: 29197830; PubMed Central PMCID: PMCPMC5779035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.LaCroix A.Z., Bellettiere J., Rillamas-Sun E., Di C., Evenson K.R., Lewis C.E. Association of light physical activity measured by accelerometry and incidence of coronary heart disease and cardiovascular disease in older women. JAMA Netw. Open. 2019;2(3) doi: 10.1001/jamanetworkopen.2019.0419. e190419. Epub 2019/03/16. PubMed PMID: 30874775; PubMed Central PMCID: PMCPMC6484645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen T., Honda T., Chen S., Narazaki K., Kumagai S. Dose-response association between accelerometer-assessed physical activity and incidence of functional disability in older Japanese adults: a 6-year prospective study. J. Gerontol. A Biol. Sci. Med. Sci. 2020 doi: 10.1093/gerona/glaa046. Epub 2020/03/07. PubMed PMID: 32134454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Manini T.M., Everhart J.E., Patel K.V., Schoeller D.A., Colbert L.H., Visser M. Daily activity energy expenditure and mortality among older adults. Jama. 2006;296(2):171–179. doi: 10.1001/jama.296.2.171. Epub 2006/07/13. PubMed PMID: 16835422. [DOI] [PubMed] [Google Scholar]