Abstract
Background
To evaluate the dosimetric parameters of different bone marrow sparing strategies and radiotherapy technologies and determine the optimal strategy to reduce hematologic toxicity associated with concurrent chemoradiation (cCRT) for cervical cancer.
Methods
A total of 15 patients with Federation International of Gynecology and Obsterics (FIGO) Stage IIB cervical cancer treated with cCRT were re-planned for bone marrow (BM)-sparing plans. First, we determined the optimal BM sparing strategy for intensity modulated radiotherapy (IMRT), including a BMS-IMRT plan that used total BM sparing (IMRT-BM) as the dose-volume constraint, and another plan used os coxae (OC) and lumbosacral spine (LS) sparing (IMRT-LS+OC) to compare the plan without BM-sparing (IMRT-N). Then, we determined the optimal technology for the BMS-IMRT, including fixed-field IMRT (FF-IMRT), volumetric-modulated arc therapy (VMAT), and helical tomotherapy (HT). The conformity and homogeneity of PTV, exposure volume of OARs, and efficiency of radiation delivery were analyzed.
Results
Compared with the IMRT-N group, the average volume of BM that received ≥10, ≥20, ≥30, and ≥40 Gy decreased significantly in both two BM-sparing groups, especially in the IMRT-LS+OC group, meanwhile, two BMS-IMRT plans exhibited the similar effect on PTV coverage and other organs at risk (OARs) sparing. Among three common IMRT techniques in clinic, HT was significantly less effective than VMAT and FF-IMRT in the aspect of BM-Sparing. Additionally, VMAT exhibited more efficient radiation delivery.
Conclusion
We recommend the use of VMAT with OC and LS as separate dose-volume constraints in cervical cancer patients aiming at reducing hematologic toxicity associated with cCRT, especially in developing countries.
Keywords: cervical cancer, bone marrow sparing, helical tomotherapy (HT), volume-modulated arc therapy (VMAT), intensity-modulated radiotherapy (IMRT)
Background
Cervical cancer is the fourth most commonly diagnosed malignancy and also the fourth most frequent cause of cancer-related mortality in women worldwide (1). The cisplatin-based cCRT has been accepted as a standard treatment for most cervical cancer patients since 1999 (2). In comparison with radiation therapy (RT) alone, cCRT increases tumor control and improves patients’ prognosis (3, 4). However, the incidence of acute hematological toxicity also increases (5). Therefore, hematologic toxicity, which may result in treatment breaks and poor prognosis of patients (6–8), is a significant clinical interest during the duration of cCRT in cervical cancer patients.
Previous studies demonstrate that the occurrence of hematologic toxicity is associated with the volume of irradiated pelvic bone marrow (BM) (9–11). Therefore, BM-sparing IMRT is considered an effective strategy to reduce hematologic toxicity in pelvic IMRT (9). More recently, studies have focused on functionally active BM sparing using [18F] fluoro-2-deoxy-2-D-glucose (FDG) and 3’-deoxy-3’-[18F] fluorothymidine (FLT) positron emission tomography/CT (PET/CT) (12–15). However, functional imaging is expensive and not universally available, especially in developing countries. Therefore, we explored the optimal dose limitation strategy and radiotherapy technology in BM-sparing IMRT for cervical cancer patients based on the current RT conditions available in most developing countries.
Previous studies have shown the IMRT plans using the lumbosacral spine (LS) and os coxae (OC) as the dose-volume constraints showed the best sparing of BM (16). Currently, the radiotherapy technologies commonly used in the treatment of cervical cancer include FF-IMRT, VMAT, and HT. Therefore, the present study aims to answer 1) which BM sparing strategy (e.g., the total BM in pelvic or BM as OC and LS separately) is more beneficial BM-sparing IMRT for cervical cancer patients? and 2) which radiotherapy technology is more effective and efficient in BM-sparing IMRT for patients with cervical cancer, FF-IMRT, VMAT, or HT?
Methods
Patients and Imaging Data
A total of 15 patients diagnosed with FIGO Stage IIB cervical cancer and treated with cCRT in our hospital between June 1st 2019 and July 30th 2019 were selected for the present study, and patients’ information is shown in Table 1 . All the patients undergone external beam radiotherapy (EBRT), brachytherapy, and concurrent chemotherapy. Before EBRT, all the patients were scanned using a Philips 16-slice Brilliance big bore computed tomography scanner (Philips Medical Systems, Amsterdam, Netherlands) with 5 mm slice thickness images, collected from the upper border of L2 vertebra to the region of 5 cm below the ischial tuberosities. All the patients were immobilized with thermoplastic mold in a supine position with comfortably full bladder and bowel preparation prior to simulation (after bladder emptying, patients were requested to drink 800 ml of water with 40 ml 60% Meglumine Diatrizoate 1 h before treatment and hold their urine). The CT scan images were transmitted into the Pinnacle3 9.10 (Philips Medical Systems, Cleveland, USA) planning system for targets and OARs contouring. The CT scan images and RT structures were transmitted to the Monaco 5.11 (Elekta AB, Stockholm, Sweden) planning system and Tomotherapy planning system (TomoTherapy Inc., Madison, WI) for radiotherapy planning design.
Table 1.
Clinicopathological characteristics of the patients with cervical squamous cell carcinoma.
| Variables | No. Patients (N = 15) |
|---|---|
| Age(years) | |
| <45 | 9 |
| ≥45 | 6 |
| SCC-Ag value | |
| 1.5–10 | 11 |
| >10 | 4 |
| Tumor size(cm) | |
| ≤4 | 10 |
| >4 | 5 |
| Deep stromal invasion | |
| No | 4 |
| Yes | 11 |
Target and Normal Tissue Delineation
Targets and OARs were delineated by the same associated chief physician. Delineation was performed according to the recommendations of the Radiation Therapy Oncology Group (RTOG) 0418 protocol and the International Commission on Radiation Units and Measurements reports (ICRU) Report 62. The clinical target volume (CTV) was defined as regions considered to embrace potential microscopic disease, including the gross tumor volume (GTV), cervix, parametria, uterus, uterosacral ligaments, sufficient vaginal margin from the gross disease (at least 3 cm), presacral region, and regional lymph nodes (e.g., common, internal, and external iliac, obturator, and presacral nodal basins). On account of the application of image guidance technique during radiotherapy, the target volume (PTV) expanded a 5 mm margin by CTV.
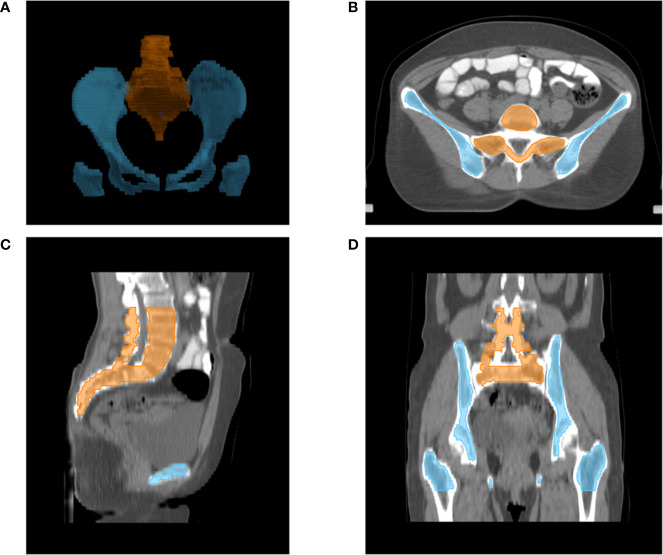
The following OARs were delineated as avoidance structures: bladder, small intestine, rectum, spinal cord, femoral heads, BM, OC, LS. Total BM was contoured from the centrum 2 cm above the upper boundary of PTV to the ischial tuberosities, including the pelvis, L4-5 centrum, and sacrum. We only contoured the marrow cavity of the intramedullary low-density area, which contains most of the hematopoietically active bone marrow, based on the bone window/level of CT simulation in pinnacle 9.10 planning system. And as reported previously (16), the total BM was also divided into two parts in this study: OC—defined as the region extending from the iliac crests to the ischial tuberosities comprising the ilium, pubis, ischium, and acetabula but not containing the femoral heads; and LS—extending from centrum 2 cm above the upper boundary of PTV to the coccyx. The contour of marrow cavity was shown in Figure 1 .
Figure 1.
Typical figures showing contours for bone marrow cavity of pelvic: Digital reconstruction (A) of pelvic bone cavity of LS (orange line) and OC (blue line); An axial (B), sagittal (C) and coronal (D) figure for inner cavity of LS (orange line) and OC (blue line).
RT Planning
All patients were treated with IMRT of 45.0 Gy in 25 daily fractions to PTV. In all plans, the PTV had the highest priority. The standard for the acceptance of the plan was that at least 95% volume of the PTV received 100% of the prescription dose, meanwhile the maximal dose of the PTV should be <110% of the prescribed dose. Dose-volume constraints for OARs were listed in Table 2 .
Table 2.
The dose-volume constraints of normal tissues in cervical cancer.
| Structures | Dose-volume constraints |
|---|---|
| Small bowel | V40 < 30%, Dmax < 48Gy |
| Rectum | V40 < 40%, Dmax < 48Gy |
| Bladder | V40 < 40% |
| Femoral head | V40 < 5% |
| Spinal cord | Dmax < 40 Gy |
| BM | V10 < 80%, V20 < 60%, V30 < 40% |
| OC | V10 < 80%, V20 < 50%, V30 < 30% |
| LS | V10 < 90%, V20 < 70%, V30 < 40% |
Radiotherapy Technologies
FF-IMRT plans were used to determine which between BM-sparing strategy is more beneficial in BM-sparing IMRT for patients with cervical cancer. And then we also confirmed the most effective and efficient technology among FF-IMRT, VMAT, and HT.
Fixed-Field IMRT Plan
The fixed-field IMRT plans were done in the Monaco 5.11 planning system and used 6 MV x-ray of a versa HD linear accelerator (Elekta Ltd., Crawley, UK). FF-IMRT plans were generated using nine evenly distributed coplanar fields with the gantry angles of 200°/240°/280°/320°/0°/40°/80°/120°/160°, and 25 control points were set in each beam. All FF-IMRT plans were calculated using Monte Carlo algorithm and the DMLC (sliding window) technique.
VMAT Plan
Similar to the FF-IMRT plans, the VMAT plans were done in the Monaco 5.11 planning system, used 6 MV x-ray of a versa HD linear accelerator, and computed using the Monte Carlo algorithm. The VMAT plans were designed using one beam with two full arcs, and there was 150 control points were found in each arc. The optimization objectives of the VMAT plans were the same as those of the FF-IMRT plans.
HT Plan
The DICOM images and RT structures were transferred to the tomotherapy planning system (Accuray Inc., Madison, WI, USA) for HT plans with 6MV photon beams. The beamlet calculation parameters included a field width of 2.5 cm, pitch value of 0.287, modulation factor of 3, and a normal dose calculation grid.
Plan Evaluation
The dose volume histograms (DVHs) obtained from the PTV and other contoured OARs were analyzed. Dosimetric parameters, including D98% (the dose received 98% volume of the PTV), D50%, D2%, the mean dose (Dmean), conformity index (CI), and homogeneity index (HI), were quantified from the PTV. CI was defined to evaluate the conformity of prescribed dose distribution (17).
Here Vt,ref, Vt, and Vref denoted the target volume that receives the prescribed dose, the target volume, and the total volume covered by the prescribed dose, respectively. The CI ranges from 0 to 1, in which a higher CI represents better conformity. According to ICRU report NO.83 (18), the HI was calculated as follows:
The smaller value of HI indicates better homogeneity of the target volume.
For OARs: Data analysis was carried out for the V10 (the OAR volume received the dose of 10 Gy), V20, V30, V40, Dmean, and Dmax. Treatment time, including the time for radiation delivery and gantry rotation, was also collected and compared.
Treatment Regimen
EBRT was delivered by FF-IMRT, VMAT, or HT, and the total dosage of EBRT was 45 Gy (applied in daily fractions of 1.8 Gy, five fractions weekly). Patients also received brachytherapy using Ir192 radioactivity (high-dose rate) with a total dosage of 30–36 Gy for each A point (5–6 fractions at 6 Gy per fraction) once or twice weekly. As a result of the dose in brachytherapy has a sharp decline, its dose was not included in the dosimetric analysis. Patients were also received 30–40 mg/m2 of cisplatin/nedaplatin once a week continuously during EBRT, beginning at the first day of radiation. The course of cCRT lasted 6–8 weeks.
Statistical Methods
Each treatment plan was normalized to the same coverage of 95% of the PTV with the prescribed dose to maintain the comparability of the results. Then, the dose-volume histogram (DVH) parameters of PTV and OARs were analyzed using paired t test. Statistical analyses were performed using SPSS version 16.0 (SPSS, Chicago, IL), and P <0.05 was considered statistically significant.
Results
The median age of all the included patients is 45 years (38–69 years). The mean volume values for PTV were 1,095.41 ± 135.49 cc. The mean volume values for the small intestine, rectum, and bladder were 1,471.06 ± 323.10, 64.85 ± 21.79, and 262.41 ± 148.88 cc, respectively. The mean volume value for the bone marrow was 283.34 ± 32.64 cc for LS and 436.06 ± 63.02 cc for OC, respectively.
Comparison of IMRT-N, IMRT-BM, and IMRT-LS+OC
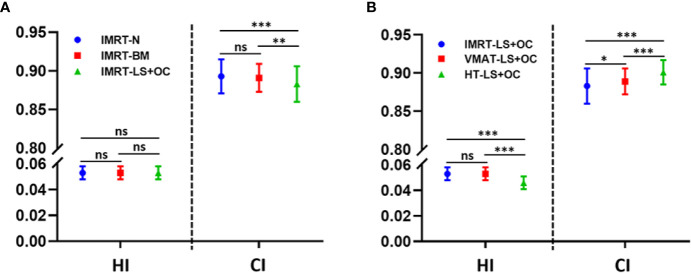
PTV dosimetric parameters and comparisons among the two BM-sparing strategies and nomal IMRT plans were summarized in Table 3 . Compared with the IMRT-N, the IMRT-BM plans have the similar CI and HI, and the IMRT-LS+OC plans have similar HI and a slightly lower CI ( Figure 2A ), which is only approximately 1.12% (IMRT-LS+OC Vs. IMRT-N: 0.883 ± 0.023 Vs. 0.893 ± 0.022, P < 0.001), indicating a slightly poorer conformal dose distribution to the PTV. Also compared with IMRT-N, the mean PTV dose increased in the IMRT-LS+OC with the differences within 0.20% (IMRT-LS+OC Vs. IMRT-N: 0.883 ± 0.023 Vs. 0.893 ± 0.022, P < 0.001). Although a statistical difference in Dmean and CI between IMRT-N and IMRT-LS+OC was observed, the difference of the absolute value is very small and the CI in all these three types of plans is ideal. In general, all these three types of plans maintained the coverage of PTV, with 95% of the total volume receiving 100% of the prescribed dose ( Figure 3 ).
Table 3.
Dose-volume histogram comparisons for PTV and main OARs in IMRT plans.
| OARs | IMRT-N | IMRT-BM | IMRT-LS+OC | P* | ||
|---|---|---|---|---|---|---|
| IMRT-BM VS IMRT-N | IMRT-LS+OC VS IMRT-N | IMRT-LS+OC VS IMRT-BM | ||||
| PTV | ||||||
| Dmean (Gy) | 45.95 ± 0.11 | 45.96 ± 0.07 | 46.04 ± 0.14 | 0.253 | 0.001 | 0.005 |
| HI | 0.053 ± 0.005 | 0.053 ± 0.005 | 0.053 ± 0.005 | 0.334 | 1.000 | 0.334 |
| CI | 0.893 ± 0.022 | 0.891 ± 0.018 | 0.883 ± 0.023 | 0.384 | <0.001 | 0.006 |
| Bone marrow (BM) | ||||||
| V10 (%) | 86.37 ± 2.50 | 84.22 ± 1.48 | 80.99 ± 2.10 | 0.003 | <0.001 | <0.001 |
| V20 (%) | 72.30 ± 2.34 | 65.54 ± 2.13 | 58.26 ± 1.10 | <0.001 | <0.001 | <0.001 |
| V30 (%) | 50.44 ± 3.24 | 43.96 ± 1.76 | 35.97 ± 1.32 | <0.001 | <0.001 | <0.001 |
| V40 (%) | 21.04 ± 2.69 | 18.66 ± 2.04 | 14.71 ± 1.47 | <0.001 | <0.001 | <0.001 |
| Dmean (Gy) | 27.92 ± 0.86 | 26.12 ± 0.36 | 24.29 ± 2.55 | <0.001 | <0.001 | 0.025 |
| Small intestine | ||||||
| V10 (%) | 82.84 ± 4.60 | 83.04 ± 4.57 | 82.12 ± 4.25 | 0.276 | 0.056 | 0.013 |
| V20 (%) | 60.56 ± 6.29 | 63.43 ± 6.40 | 65.05 ± 5.62 | <0.001 | <0.001 | 0.027 |
| V30 (%) | 37.29 ± 7.38 | 37.73 ± 7.13 | 37.58 ± 7.37 | 0.164 | 0.528 | 0.745 |
| V40 (%) | 17.84 ± 5.52 | 18.18 ± 5.60 | 18.29 ± 5.44 | 0.007 | 0.026 | 0.513 |
| Dmax (Gy) | 47.00 ± 0.28 | 47.06 ± 0.19 | 47.16 ± 0.31 | 0.194 | 0.004 | 0.130 |
| Rectum | ||||||
| V10 (%) | 97.35 ± 2.96 | 97.68 ± 2.77 | 97.66 ± 2.88 | 0.018 | 0.019 | 0.839 |
| V20 (%) | 93.73 ± 3.70 | 93.94 ± 3.40 | 93.52 ± 3.30 | 0.327 | 0.404 | 0.072 |
| V30 (%) | 75.97 ± 3.99 | 76.12 ± 3.21 | 74.85 ± 3.91 | 0.877 | 0.256 | 0.177 |
| V40 (%) | 34.14 ± 4.99 | 34.56 ± 5.83 | 35.96 ± 6.85 | 0.602 | 0.125 | 0.212 |
| Dmax (Gy) | 45.46 ± 0.49 | 45.40 ± 0.50 | 45.70 ± 0.52 | 0.481 | 0.013 | <0.001 |
| Bladder | ||||||
| V10 (%) | 98.64 ± 1.73 | 98.15 ± 2.08 | 98.19 ± 1.98 | 0.261 | 0.140 | 0.830 |
| V20 (%) | 82.34 ± 2.58 | 82.41 ± 3.36 | 82.41 ± 3.69 | 0.891 | 0.904 | 0.998 |
| V30 (%) | 63.73 ± 3.30 | 64.55 ± 1.93 | 64.57 ± 2.01 | 0.324 | 0.385 | 0.965 |
| V40 (%) | 43.37 ± 4.40 | 43.20 ± 5.71 | 43.51 ± 5.21 | 0.618 | 0.669 | 0.471 |
| Spinal Cord | ||||||
| Dmax (Gy) | 34.10 ± 3.03 | 28.40 ± 4.88 | 17.70 ± 3.74 | <0.001 | <0.001 | <0.001 |
| Femoral head-L | ||||||
| V10 (%) | 76.19 ± 7.57 | 77.54 ± 8.25 | 78.96 ± 9.71 | 0.434 | 0.211 | 0.561 |
| V20 (%) | 38.42 ± 6.18 | 38.10 ± 5.55 | 37.61 ± 5.73 | 0.802 | 0.538 | 0.528 |
| V30 (%) | 14.41 ± 5.33 | 13.56 ± 5.23 | 11.74 ± 4.95 | 0.152 | 0.006 | 0.043 |
| V40 (%) | 0.794 ± 1.306 | 0.687 ± 1.290 | 0.687 ± 1.308 | 0.294 | 0.676 | 0.998 |
| Femoral head-R | ||||||
| V10 (%) | 73.84 ± 8.25 | 75.77 ± 6.80 | 77.25 ± 8.59 | 0.356 | 0.034 | 0.351 |
| V20 (%) | 33.70 ± 5.84 | 33.63 ± 6.81 | 34.06 ± 6.52 | 0.945 | 0.790 | 0.615 |
| V30 (%) | 11.93 ± 5.64 | 11.05 ± 5.24 | 10.44 ± 5.59 | 0.130 | 0.019 | 0.236 |
| V40 (%) | 1.043 ± 1.819 | 0.821 ± 1.645 | 0.564 ± 1.416 | 0.401 | 0.199 | 0.097 |
*P value was computed by paired t test.
Figure 2.
Conformity index (CI) and homogeneity index (HI) for planning target volume (PTV) with different BM-sparing strategies (A) and different radiotherapy technologies (B). * P < 0.05, ** P < 0.01, *** P < 0.001. ns: P > 0.05, no statistical significance.
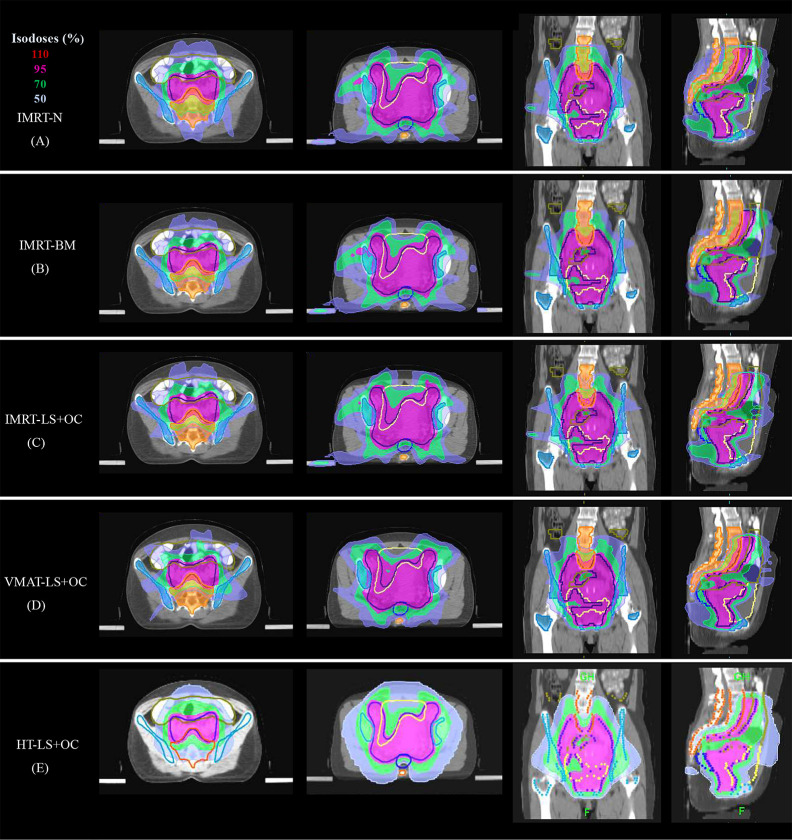
Figure 3.
Typical dose distributions of the five plans in cervical cancer. (A) IMRT-N, (B) IMRT-BM, (C) IMRT-LS+OC, (D) VMAT-LS+OC, and (E) HT-LS+OC plans.
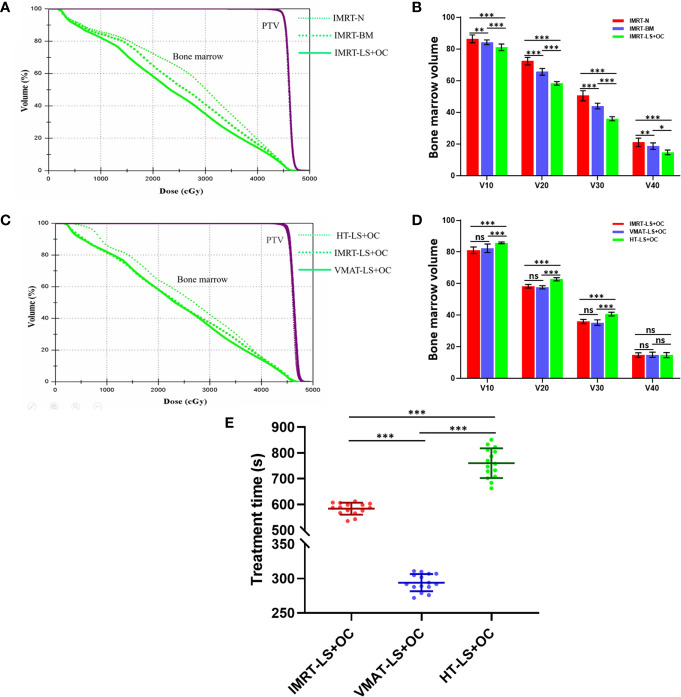
The dose parameters of the BM are also listed in Table 3 , and the typical dose-volume histograms for BM are shown in Figures 4A, B . Compared with the IMRT-N group, the average volume of BM receiving ≥10, ≥20, ≥30, and ≥40 Gy and the mean dose decreased significantly in the two BM-sparing groups, especially in the IMRT-LS+OC group. The volume reductions are presented as follows: 2.49 and 6.23% for V10 (IMRT-BM Vs. MRT-N: 84.22 ± 1.48 Vs. 86.37 ± 2.50%, P = 0.003; IMRT-LS+OC Vs. IMRT-N: 80.99 ± 2.10 Vs.86.37 ± 2.50%, P < 0.001), 9.35 and 19.42% for V20 (IMRT-BM Vs. IMRT-N: 65.54 ± 2.13 Vs.72.30 ± 2.34%, P < 0.001; IMRT-LS+OC Vs. IMRT-N: 58.26 ± 1.10 Vs 72.30 ± 2.34%, P < 0.001), 12.85 and 28.69% for V30 (IMRT-BM Vs. IMRT-N: 43.96 ± 1.76 Vs. 50.44 ± 3.24%, P < 0.001; IMRT-LS+OC Vs. IMRT-N: 35.97 ± 1.32 Vs. 50.44 ± 3.24%, P < 0.001), and 11.31 and 30.09% for V40 (IMRT-BM Vs. IMRT-N: 18.66 ± 2.04 Vs. 21.04 ± 2.69%, P < 0.001; IMRT-LS+OC Vs. IMRT-N: 14.71 ± 1.47 Vs. 21.04 ± 2.69%, P < 0.001), and the mean dose reduction for bone marrow was 6.45 and 13.00% (IMRT-BM Vs. IMRT-N: 26.12 ± 0.36 Vs. 27.92 ± 0.86%, P < 0.001; IMRT-LS+OC Vs. IMRT-N: 24.29 ± 2.55 Vs. 27.92 ± 0.86%, P < 0.001).
Figure 4.
The comparison of planning target volume, bone marrow volume, and treatment time in BM-sparing IMRT. Typical dose-volume histograms for planning target volume (purple) and bone marrow (green) (A+C), histogram for bone marrow volume (B+D) with three different dose limitation strategies for pelvic radiotherapy: IMRT-N, IMRT-BM, and IMRT-LS+OC and three different radiotherapy technologies: IMRT-LS+OC, VMAT-LS+OC, and HT-LS+OC. Typical scatter diagram for treatment time (E) with three different radiotherapy technologies. * P < 0.05, ** P < 0.01, *** P < 0.001. ns: P > 0.05, no statistical significance.
The dose parameters of other OARs were also listed in Table 3 . According to our results, not all the plans achieved our dose-volume constraints of OARs, the main reason is that the priority of PTV is the highest in the plan parameter setting. In the case that the OARs cannot achieve the preset conditions, the OARs will compromise to ensure the CI and HI of PTV.
The mean V20 and V40 of small intestine and V10 of rectum, were statistically and significantly increased by the IMRT-BM and IMRT-LS+OC plans compared with the IMRT-N plan. However, the difference of the absolute value is very small and still within our dose-volume constraint. On the contrary, Dmax of the spinal cord showed a significant decrease in these two BM-sparing plans, especially in the IMRT-LS+OC (IMRT-BM Vs. IMRT-N: 28.40 ± 4.88 Vs. 34.10 ± 3.03%; IMRT-LS+OC Vs. IMRT-N: 17.70 ± 3.74 Vs. 34.10 ± 3.03%, both P < 0.001), which may be caused by the dose limitation of the adjacent BM.
Comparison of HT, VMAT, and FF-IMRT
Subsequently, we further investigated which radiotherapy technology is more effective in IMRT-LS+OC for patients with cervical cancer.
With regard to conformal and homogeneous dose distribution to the PTV target dose distribution, a significant benefit from the HT plans with lowest HI and highest CI were observed, and FF-IMRT and VMAT were comparable ( Table 4 and Figure 2B ). However, HT demonstrated a more inferior sparing in the BM considering the V10, V20, and V30 of BM compared with FF-IMRT and VMAT (all P < 0.001) ( Figures 4C, D ). Additionally, all these three radiotherapy techniques resulted in sufficient sparing of the OARs, and we have listed all the average doses to the OARs in Table 4 . The treatment time was also listed in Table 4 and Figure 4E , the mean treatment time of FF-IMRT, VMAT, and HT were 583.53 ± 22.88, 294.13 ± 12.55, and 760.33 ± 53.48 s respectively. In comparison with conventional FF-IMRT and HT, the mean treatment time of VMAT was greatly decreased by 49.59 and 61.32% (both P < 0.001).
Table 4.
Dosimetric parameters for PTV and main OARs in FF-IMRT, VMAT, and TOMO plans.
| Parameters | FF-IMRT | VMAT | TOMO | P* | ||
|---|---|---|---|---|---|---|
| VMAT Vs. FF-IMRT | TOMO Vs. FF-IMRT | TOMO Vs. VMAT | ||||
| PTV | ||||||
| Dmean (Gy) | 46.04 ± 0.14 | 46.05 ± 0.09 | 46.17 ± 0.20 | 0.945 | 0.076 | 0.035 |
| HI | 0.053 ± 0.005 | 0.053 ± 0.005 | 0.046 ± 0.005 | 1.000 | <0.001 | <0.001 |
| CI | 0.883 ± 0.023 | 0.889 ± 0.017 | 0.901 ± 0.016 | 0.014 | <0.001 | <0.001 |
| Bone marrow | ||||||
| V10 (%) | 80.99 ± 2.10 | 82.30 ± 2.67 | 85.73 ± 0.54 | 0.013 | <0.001 | <0.001 |
| V20 (%) | 58.26 ± 1.10 | 57.60 ± 1.00 | 62.69 ± 1.00 | 0.061 | <0.001 | <0.001 |
| V30 (%) | 35.97 ± 1.32 | 35.27 ± 1.73 | 40.67 ± 1.12 | 0.062 | <0.001 | <0.001 |
| V40 (%) | 14.71 ± 1.47 | 14.91 ± 1.63 | 14.70 ± 1.63 | 0.450 | 0.956 | 0.334 |
| Dmean (Gy) | 24.49 ± 2.55 | 23.86 ± 0.27 | 25.56 ± 0.68 | 0.350 | 0.139 | <0.001 |
| Small intestine | ||||||
| V10 (%) | 82.12 ± 4.25 | 83.74 ± 4.77 | 91.45 ± 4.32 | <0.001 | <0.001 | <0.001 |
| V20 (%) | 65.05 ± 5.62 | 61.82 ± 6.22 | 61.59 ± 6.73 | <0.001 | <0.001 | 0.758 |
| V30 (%) | 37.58 ± 7.37 | 37.63 ± 6.41 | 36.82 ± 7.74 | 0.894 | 0.137 | 0.118 |
| V40 (%) | 18.29 ± 5.44 | 18.16 ± 5.58 | 16.53 ± 5.39 | 0.460 | <0.001 | 0.002 |
| Dmax (Gy) | 47.16 ± 0.31 | 47.21 ± 0.29 | 46.98 ± 0.32 | 0.541 | 0.06 | 0.005 |
| Rectum | ||||||
| V10 (%) | 97.66 ± 2.88 | 98.28 ± 2.43 | 99.03 ± 1.74 | 0.001 | 0.010 | 0.071 |
| V20 (%) | 93.52 ± 3.30 | 95.30 ± 3.53 | 83.12 ± 7.90 | <0.001 | <0.001 | <0.001 |
| V30 (%) | 74.85 ± 3.91 | 75.95 ± 3.75 | 54.06 ± 7.59 | 0.378 | <0.001 | <0.001 |
| V40 (%) | 35.96 ± 6.85 | 38.20 ± 4.57 | 27.26 ± 5.40 | 0.208 | <0.001 | <0.001 |
| Dmax (Gy) | 45.70 ± 0.52 | 43.90 ± 7.57 | 46.45 ± 0.53 | 0.360 | <0.001 | 0.202 |
| Bladder | ||||||
| V10 (%) | 98.19 ± 1.98 | 99.29 ± 1.25 | 100 ± 0.00 | 0.014 | 0.003 | 0.045 |
| V20 (%) | 82.41 ± 3.69 | 85.28 ± 5.94 | 92.55 ± 10.54 | 0.025 | <0.001 | 0.008 |
| V30 (%) | 64.57 ± 2.01 | 63.39 ± 2.62 | 61.45 ± 5.20 | 0.108 | 0.023 | 0.201 |
| V40 (%) | 43.51 ± 5.21 | 42.38 ± 5.45 | 35.89 ± 4.50 | 0.039 | <0.001 | <0.001 |
| Spinal Cord | ||||||
| Dmax (Gy) | 17.70 ± 3.74 | 13.98 ± 2.32 | 22.42 ± 3.75 | <0.001 | 0.006 | <0.001 |
| Femoral head-L | ||||||
| V10 (%) | 78.96 ± 9.71 | 86.80 ± 10.09 | 98.67 ± 2.00 | 0.005 | <0.001 | 0.001 |
| V20 (%) | 37.61 ± 5.73 | 29.48 ± 8.31 | 66.52 ± 9.96 | 0.002 | <0.001 | <0.001 |
| V30 (%) | 11.74 ± 4.95 | 4.98 ± 3.30 | 29.41 ± 11.35 | <0.001 | <0.001 | <0.001 |
| V40 (%) | 0.687 ± 1.308 | 0.089 ± 0.204 | 0.235 ± 0.593 | 0.078 | 0.221 | 0.349 |
| Femoral head-R | ||||||
| V10 (%) | 77.25 ± 8.59 | 82.96 ± 7.79 | 98.92 ± 1.99 | 0.043 | <0.001 | <0.001 |
| V20 (%) | 34.06 ± 6.52 | 26.89 ± 12.49 | 66.22 ± 9.23 | 0.064 | <0.001 | <0.001 |
| V30 (%) | 10.44 ± 5.59 | 4.29 ± 5.32 | 25.72 ± 10.61 | <0.001 | <0.001 | <0.001 |
| V40 (%) | 0.564 ± 1.416 | 0.066 ± 0.144 | 0.058 ± 0.201 | 0.161 | 0.190 | 0.888 |
| Treatment time (s) | 583.53 ± 22.88 | 294.13 ± 12.55 | 760.33 ± 53.48 | <0.001 | <0.001 | <0.001 |
*P value was computed by paired t test.
Discussion
Our study explored the optimal bone marrow sparing strategy and radiotherapy technology in BM-sparing IMRT for cervical cancer patients who receive the cCRT. Our results demonstrated that BM-sparing IMRT plan with OC and LS as separate dose-volume constraints achieved the best BM-sparing without compromising target volume dose coverage and increasing the radiation dose to other OARs. We also demonstrated that all techniques achieved adequate coverage of PTV, however, VMAT planning yielded better BM-Sparing and shorter estimated treatment times when compared to HT planning.
It is known that BM is composed of both hematopoietically active “red” marrow and inactive “yellow” marrow, and approximately 50% of hematopoietically active BM lies within the lumbar sacrum, ilium, ischium, pubis, and proximal femur (19), where are just adjacent to the pelvic EBRT field of cervical cancer. Meantime, BM is extremely sensitive to irradiation, haematopoietic stem cells is damaged and BM microenvironment is modified adversely even at low doses (20–23). Previous studies indicated that the mean radiation dose and the volume that receives lower dose irradiation of the pelvic bone marrow were relative to hematologic toxicity (24–27). With the development of radiotherapy technology, IMRT that uses multiple beam angles or arcs, can reduce irradiation dose to OARs, which is correlated with reduced acute and late adverse events (AEs) (28–30). However, the irradiation of a large volume of BM is unavoidable during pelvic IMRT.
As most of previous studies focusing on PET-guided functional BM demonstrated that most of the functional BMs were located in the marrow cavity (12–15), OC, LS, and total BM were delineated on the basis of the marrow cavity of the intramedullary low-density area in our current study considering limited medical resources and treatment cost in our developing countries. Our results demonstrated that BM-sparing IMRT plan with OC and LS as separate dose-volume constraints achieved the best BM-sparing, with the lowest volume of BM receiving ≥10, ≥20, ≥30, and ≥40 Gy. With the rapid development of the field of artificial intelligence, the program of automatic delineation of OARs is becoming mature. We applied the program of automatic delineation of OARs in the MIM version 6.7 system (MIM Software, Cleveland, USA) through a database of 50 patients and delineated the OC and LS relatively simple. Therefore, we recommended to introduce OC and LS as the independent OARs and use dose-volume constraints in BM-sparing IMRT. These results were similar to previous study on BM-IMRT in cervical cancer of Prof. Bao (16), and the main innovations of our study are the delineation method of bone marrow and the dose constraint method of LS and OC.
Highly conformal radiation techniques have dramatically improved, in which the most representative ones are HT and VMAT. Compared with FF-IMRT plan, HT plan has a highly conformal dose distribution and reduces the high-dose volume of OARs close to the target (31, 32) while VMAT plans have less monitor units (MUs) and treatment time. Our results from the dosimetric comparison of these technologies in BM-sparing IMRT demonstrated that all plans met our clinical demand, and HT demonstrated the highest CI and lowest HI but the inferior sparing of low-dose radiation in BM (e.g., V10, V20, and V30). Meanwhile, compared with FF-IMRT and HT, the main advantage of VMAT was that it spent relatively less time on radiation delivery, thereby reducing patients’ discomfort and the probability of patents’ moving during treatment. Collectively, these results indicate that VMAT can be considered the most optimized and effective technology in BM-sparing IMRT of cervical cancer. To the best of our knowledge, no published data compare the planning parameters of HT and VAMT vs. FF-IMRT in terms of the effectiveness and efficiency of BM-sparing IMRT in cervical cancer patients.
As we know, the medical resources are insufficient in most of the developing countries. Barriers to availability of radiotherapy in these countries are attributed primarily to costs, including equipment cost and the cost of staff education. There are about only two medical linear accelerators per million people in China. Our results demonstrated that in the BM-sparing IMRT, the mean treatment time of VMAT was greatly decreased by 49.59 and 61.32%, compared with the conventional FF-IMRT and HT. The results are consistent with previous studies in other solid cancers (33–35). Therefore, VMAT, with a better dose conformity or sparing of OARs and a shorter treatment delivery time, is more recommended in the BM-sparing IMRT for cervical cancer patients, especially in the developing countries.
Our study has some limitations. First, we generally contoured the low-density regions of the bone (marrow-cavity) as the BM based on the bone window/level of CT simulation in pinnacle 9.10 planning system, this strategy may overestimate the volume of active BM or define the active BM inadequately. Second, we used the choice of uniform outward expansion of 5mm from CTV to generate a PTV in all the cases because we used image-guided technique to reduce the positioning error. However, due to the influence of organ motion, positioning error and other factors, the generation rules of PTV of radiotherapy for cervical cancer have not reached a consensus. Lastly, this study only focused on the dosimetric advantages of BM-sparing IMRT technology at the planning and design level. Future work should focus on its clinical significance correlated with the hematologic toxicity of cervical cancer patients treated with cCRT.
Conclusion
Our study confirmed that BM-sparing IMRT plan with OC and LS as separate dose-volume constraints achieved the best BM-sparing without compromising target volume dose coverage. Moreover, VMAT exhibited the optimal BM-sparing and efficiency in all the advanced radiotherapy technologies. Therefore, VMAT with OC and LS as separate dose-volume constraints, as an effective BM-sparing IMRT, is a promising treatment option to reduce hematologic toxicity for cervical cancer patients in developing countries with limited medical resources and expenditures, not only avoids expensive functional imaging of active bone marrow but also improves the efficiency of radiotherapy.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
This study complied with the Helsinki Declaration and approval from the Ethics Committee of Harbin Medical University Cancer Hospital (Harbin, China) was obtained. All patients provided their written informed consent for the publication of their images/data.
Author Contributions
S-SY, D-YY, and Y-YZ designed the study. S-SY contoured the target and OARs. D-YY performed the design of the treatment planning. W-KY, YF, LW, J-YS, and XL collected the data. D-YY, YF, LW, Y-YZ, and S-SY wrote and revised the manuscript. Y-LB and Y-YZ polished the language. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 81872460 and 81602664), Project of precise radiotherapy spark program (2019-N-11-11), and Pandeng Project of National Cancer Center of China (NCC201808B016).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank all the people who had participated in this study.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. New Engl J Med (1999) 340(15):1144–53. 10.1056/NEJM199904153401502 [DOI] [PubMed] [Google Scholar]
- 3. Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. New Engl J Med (1999) 340(15):1154–61. 10.1056/NEJM199904153401503 [DOI] [PubMed] [Google Scholar]
- 4. PETERS IIIW. Concurrent chemotherapy and pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol (2000) 18:1606–13. 10.1200/JCO.2000.18.8.1606 [DOI] [PubMed] [Google Scholar]
- 5. Duenas-González A, Zarbá JJ, Patel F, Alcedo JC, Beslija S, Casanova L, et al. open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol (2011) 29(13):1678–85. 10.1200/JCO.2009.25.9663 [DOI] [PubMed] [Google Scholar]
- 6. Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet (9284) 2001) 358:781–6. 10.1016/S0140-6736(01)05965-7 [DOI] [PubMed] [Google Scholar]
- 7. Torres MA, Jhingran A, Thames HD, Jr., Levenback CF, Bodurka DC, Ramondetta LM, et al. Comparison of treatment tolerance and outcomes in patients with cervical cancer treated with concurrent chemoradiotherapy in a prospective randomized trial or with standard treatment. Int J Radiat Oncol Biol Phys (2008) 70(1):118–25. 10.1016/j.ijrobp.2007.05.028 [DOI] [PubMed] [Google Scholar]
- 8. Bazan JG, Luxton G, Kozak MM, Anderson EM, Hancock SL, Kapp DS, et al. Impact of chemotherapy on normal tissue complication probability models of acute hematologic toxicity in patients receiving pelvic intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys (2013) 87(5):983–91. 10.1016/j.ijrobp.2013.09.017 [DOI] [PubMed] [Google Scholar]
- 9. Mell LK, Schomas DA, Salama JK, Devisetty K, Aydogan B, Miller RC, et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys (2008) 70(5):1431–7. 10.1016/j.ijrobp.2007.08.074 [DOI] [PubMed] [Google Scholar]
- 10. Wan J, Liu K, Li K, Li G, Zhang Z. Can dosimetric parameters predict acute hematologic toxicity in rectal cancer patients treated with intensity-modulated pelvic radiotherapy? Radiat Oncol (2015) 10(1):1–7. 10.1186/s13014-015-0454-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Franco P, Ragona R, Arcadipane F, Mistrangelo M, Cassoni P, Rondi N, et al. Dosimetric predictors of acute hematologic toxicity during concurrent intensity-modulated radiotherapy and chemotherapy for anal cancer. Clin Trans Oncol (2017) 19(1):67–75. 10.1007/s12094-016-1504-2 [DOI] [PubMed] [Google Scholar]
- 12. Liang Y, Bydder M, Yashar CM, Rose BS, Cornell M, Hoh CK, et al. Prospective study of functional bone marrow-sparing intensity modulated radiation therapy with concurrent chemotherapy for pelvic malignancies. Int J Radiat Oncol Biol Phys (2013) 85(2):406–14. 10.1016/j.ijrobp.2012.04.044 [DOI] [PubMed] [Google Scholar]
- 13. Elicin O, Callaway S, Prior JO, Bourhis J, Ozsahin M, Herrera FG. [18F] FDG-PET standard uptake value as a metabolic predictor of bone marrow response to radiation: impact on acute and late hematological toxicity in cervical cancer patients treated with chemoradiation therapy. Int J Radiat Oncol Biol Phys (2014) 90(5):1099–107. 10.1016/j.ijrobp.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 14. Wyss JC, Carmona R, Karunamuni RA, Pritz J, Hoh CK, Mell LK. [18F] Fluoro-2-deoxy-2-d-glucose versus 3′-deoxy-3′-[18F] fluorothymidine for defining hematopoietically active pelvic bone marrow in gynecologic patients. Radiother Oncol (2016) 118(1):72–8. 10.1016/j.radonc.2015.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mell LK, Sirák I, Wei L, Tarnawski R, Mahantshetty U, Yashar CM, et al. Bone marrow-sparing intensity modulated radiation therapy with concurrent cisplatin for stage IB-IVA cervical cancer: an international multicenter phase II clinical trial (INTERTECC-2). Int J Radiat Oncol Biol Phys (2017) 97(3):536–45. 10.1016/j.ijrobp.2016.11.027 [DOI] [PubMed] [Google Scholar]
- 16. Bao Z, Wang D, Chen S, Chen M, Jiang D, Yang C, et al. Optimal dose limitation strategy for bone marrow sparing in intensity-modulated radiotherapy of cervical cancer. Radiat Oncol (2019) 14(1):118. 10.1186/s13014-019-1324-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van’t Riet A, Mak AC, Moerland MA, Elders LH, Van Der Zee W. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate. Int J Radiat Oncol Biol Phys (1997) 37(3):731–6. 10.1016/S0360-3016(96)00601-3 [DOI] [PubMed] [Google Scholar]
- 18. Hodapp N. Der ICRU-Report 83: Verordnung, Dokumentation und Kommunikation der fluenzmodulierten Photonenstrahlentherapie (IMRT). Strahlentherapie und Onkologie (2012) 188(1):97–100. 10.1007/s00066-011-0015-x [DOI] [PubMed] [Google Scholar]
- 19. Ellis R. The distribution of active bone marrow in the adult. Phys Med Biol (1961) 5(3):255. 10.1088/0031-9155/5/3/302 [DOI] [PubMed] [Google Scholar]
- 20. Blomlie V, Rofstad EK, Skjønsberg A, Tverå K, Lien HH. Female pelvic bone marrow: serial MR imaging before, during, and after radiation therapy. Radiology (1995) 194(2):537–43. 10.1148/radiology.194.2.7824737 [DOI] [PubMed] [Google Scholar]
- 21. Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys (1995) 31(5):1319–39. 10.1016/0360-3016(94)00430-S [DOI] [PubMed] [Google Scholar]
- 22. Cao X, Wu X, Frassica D, Yu B, Pang L, Xian L, et al. Irradiation induces bone injury by damaging bone marrow microenvironment for stem cells. Proc Natl Acad Sci (2011) 108(4):1609–14. 10.1073/pnas.1015350108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Forrest J, Presutti J, Davidson M, Hamilton P, Kiss A, Thomas G. A dosimetric planning study comparing intensity-modulated radiotherapy with four-field conformal pelvic radiotherapy for the definitive treatment of cervical carcinoma. Clin Oncol (2012) 24(4):e63–70. 10.1016/j.clon.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 24. Mell LK, Kochanski JD, Roeske JC, Haslam JJ, Mehta N, Yamada SD, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys (2006) 66(5):1356–65. 10.1016/j.ijrobp.2006.03.018 [DOI] [PubMed] [Google Scholar]
- 25. Albuquerque K, Giangreco D, Morrison C, Siddiqui M, Sinacore J, Potkul R, et al. Radiation-related predictors of hematologic toxicity after concurrent chemoradiation for cervical cancer and implications for bone marrow–sparing pelvic IMRT. Int J Radiat Oncol Biol Phys (2011) 79(4):1043–7. 10.1016/j.ijrobp.2009.12.025 [DOI] [PubMed] [Google Scholar]
- 26. Rose BS, Aydogan B, Liang Y, Yeginer M, Hasselle MD, Dandekar V, et al. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys (2011) 79(3):800–7. 10.1016/j.ijrobp.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu H, Zakeri K, Vaida F, Carmona R, Dadachanji KK, Bair R, et al. Longitudinal study of acute haematologic toxicity in cervical cancer patients treated with chemoradiotherapy. J Med Imaging Radiat Oncol (2015) 59(3):386–93. 10.1111/1754-9485.12297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mundt AJ, Lujan AE, Rotmensch J, Waggoner SE, Yamada SD, Fleming G, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys (2002) 52(5):1330–7. 10.1016/S0360-3016(01)02785-7 [DOI] [PubMed] [Google Scholar]
- 29. Gandhi AK, Sharma DN, Rath GK, Julka PK, Subramani V, Sharma S, et al. Early clinical outcomes and toxicity of intensity modulated versus conventional pelvic radiation therapy for locally advanced cervix carcinoma: a prospective randomized study. Int J Radiat Oncol Biol Phys (2013) 87(3):542–8. 10.1016/j.ijrobp.2012.07.050 [DOI] [PubMed] [Google Scholar]
- 30. Klopp AH, Yeung AR, Deshmukh S, Gil KM, Wenzel L, Westin SN, et al. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG oncology–RTOG 1203. J Clin Oncol (2018) 36(24):2538. 10.1200/JCO.2017.77.4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lian J, Mackenzie M, Joseph K, Pervez N, Dundas G, Urtasun R, et al. Assessment of extended-field radiotherapy for stage IIIC endometrial cancer using three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, and helical tomotherapy. Int J Radiat Oncol Biol Phys (2008) 70(3):935–43. 10.1016/j.ijrobp.2007.10.021 [DOI] [PubMed] [Google Scholar]
- 32. Engels B, De Ridder M, Tournel K, Sermeus A, De Coninck P, Verellen D, et al. Preoperative helical tomotherapy and megavoltage computed tomography for rectal cancer: impact on the irradiated volume of small bowel. Int J Radiat Oncol Biol Phys (2009) 74(5):1476–80. 10.1016/j.ijrobp.2008.10.017 [DOI] [PubMed] [Google Scholar]
- 33. Clivio A, Fogliata A, Franzetti-Pellanda A, Nicolini G, Vanetti E, Wyttenbach R, et al. Volumetric-modulated arc radiotherapy for carcinomas of the anal canal: A treatment planning comparison with fixed field IMRT. Radiother Oncol (2009) 92(1):118–24. 10.1016/j.radonc.2008.12.020 [DOI] [PubMed] [Google Scholar]
- 34. Vanetti E, Clivio A, Nicolini G, Fogliata A, Ghosh-Laskar S, Agarwal JP, et al. Volumetric modulated arc radiotherapy for carcinomas of the oro-pharynx, hypo-pharynx and larynx: a treatment planning comparison with fixed field IMRT. Radiother Oncol (2009) 92(1):111–7. 10.1016/j.radonc.2008.12.008 [DOI] [PubMed] [Google Scholar]
- 35. Bertelsen A, Hansen CR, Johansen J, Brink C. Single arc volumetric modulated arc therapy of head and neck cancer. Radiother Oncol (2010) 95(2):142–8. 10.1016/j.radonc.2010.01.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.