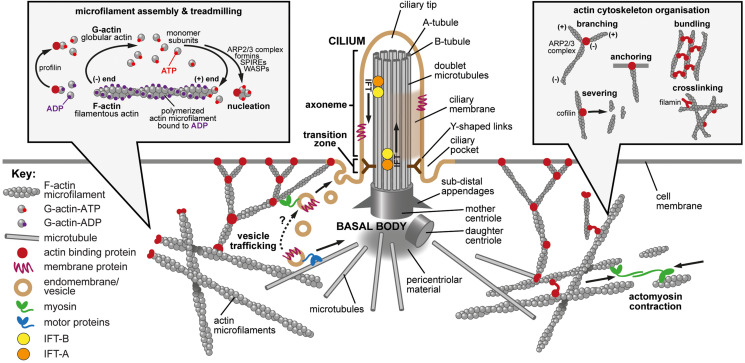
FIGURE 1.
The primary cilium, actin dynamics and the actin cytoskeleton. Main panel: The primary cilium and the actin cytoskeleton. The primary cilium forms when the mother centriole docks at the apical membrane to form the basal body. Microtubules (gray cylinders) then nucleate at the basal body to initiate formation of the axoneme with a 9+0 microtubule arrangement. The sub-distal appendages mediate microtubule anchoring, and the pericentriolar material may act as a microtubule organizing center. Y-shaped links (dark brown) connect the microtubule doublets to the ciliary membrane at the ciliary transition zone, which acts as a gate to control the entry and exit of proteins and lipids. Intraflagellar transport (IFT) protein complexes (orange and yellow) transport cargo (indicated by arrows) along the axoneme by using microtubule based motor proteins. The ciliary pocket is an endocytic membrane domain around the base of the cilium implicated in actin dynamics, the transport of membrane associated proteins to cilia and in ciliary disassembly. The ciliary tip is a source of extracellular vesicles and is involved in early ciliary disassembly through decapitation. The actin cytoskeleton modulates ciliogenesis through effects on both vesicle trafficking (lower left; tan symbols and dashed arrow) and actin cytoskeleton remodeling mediated by acto-myosin contractions (lower right; green symbols and arrows). Actin-binding proteins are indicated by dark red symbols. Inset left: Microfilament assembly and treadmilling. Actin is present in cells as both a monomer (globular, G-actin) and a polymer (filamentous, F-actin). Actin binds and can hydrolyze ATP to ADP. There is preferential additional of ATP-bound monomers to the (+) end of the polymer and ATP is hydrolyzed upon filament assembly. The preferential dissociation of ADP-bound actin results in a “treadmilling” effect, whereby the F-actin filaments exhibit net growth at their (+) ends and net dissociation at their (−) ends. Both polymerization and depolymerization require actin binding proteins. Profilin can sequester G-actin from the pool of actin monomers but can also catalyze the exchange of ADP to ATP, converting monomers to the more polymerizable ATP-bound form. Actin polymerization requires nucleating factors, such as Spire or formins, or the actin-related protein complex (ARP2/3). Actin depolymerization remains incompletely understood but members of the yeast actin depolymerization factor (ADF)/cofilin family can enhance dissociation of monomers from the (−) end. Inset right: Actin cytoskeleton organization. Many actin binding proteins can alter the arrangement and structure of F-actin. ARP2/3 can elicit F-actin branching. Members of the yeast actin depolymerization factor (ADF)/cofilin family can both enhance dissociation of G-actin monomers from the (−) end and can sever filaments to produce additional (−) ends. Filamins and various other actin binding proteins can crosslink actin to form complex networks, can anchor F-actin to membranes and can bundle actin into stress fibers.