Figure 1.
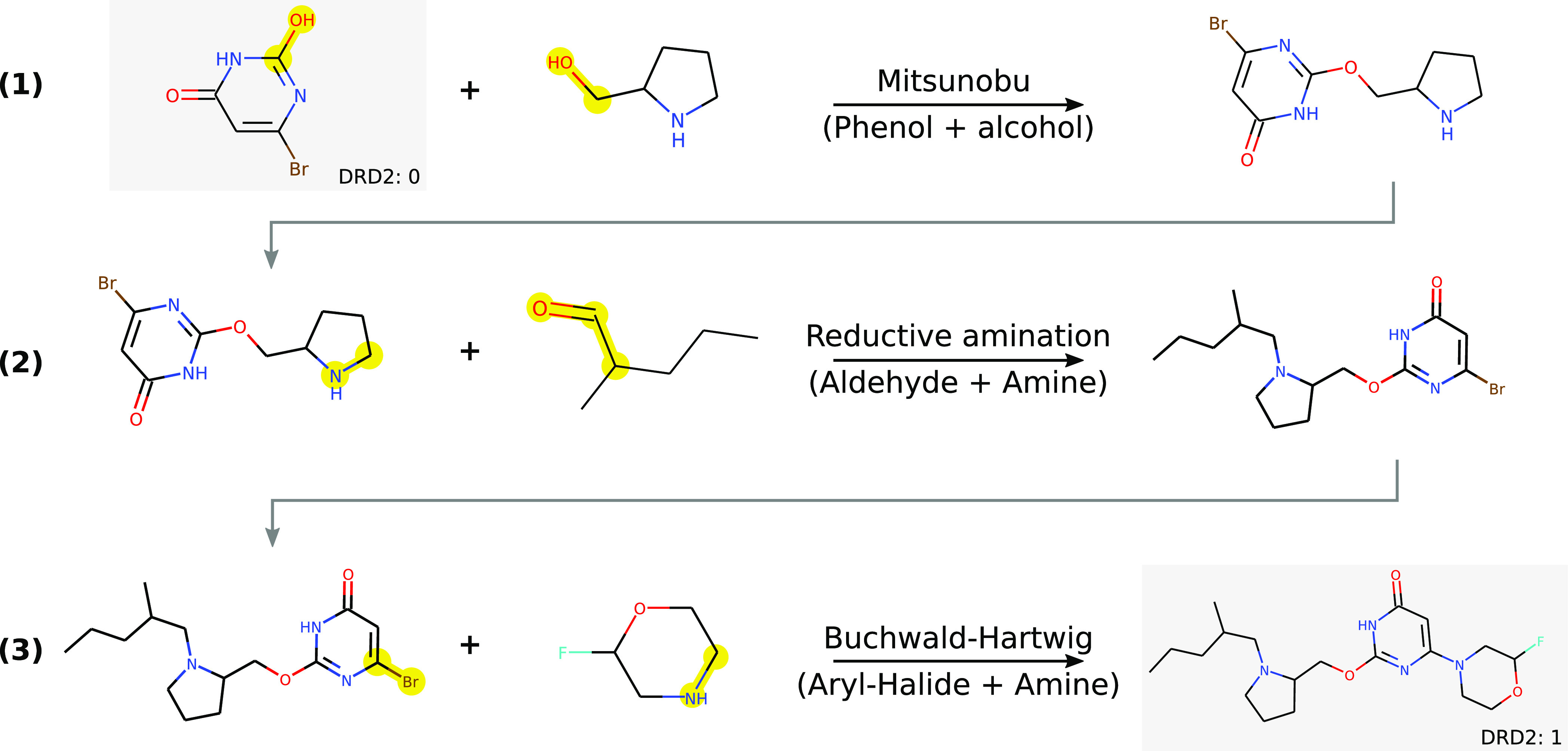
A trajectory taken by the REACTOR agent during the optimization of affinity for the dopamine receptor D2.This trajectory provides a high-level overview of a possible synthesis route for the proposed molecule in three steps: (1) a Mitsunobu reaction, (2) a reductive amination, and (3) a Buchwald–Hartwig amination. We note that, although the proposed route is theoretically feasible, it would not be the first choice for synthesis and can easily be optimized. Nevertheless, it remains an important indication of synthesizability. We also note here that the agent learns a policy that produces structures containing a pyrrolidine/piperidine moiety, which have been shown as actives against dopamine receptors.14,15
