Figure 2.
Fructose Activated Pathways That Lead to Insulin Resistance
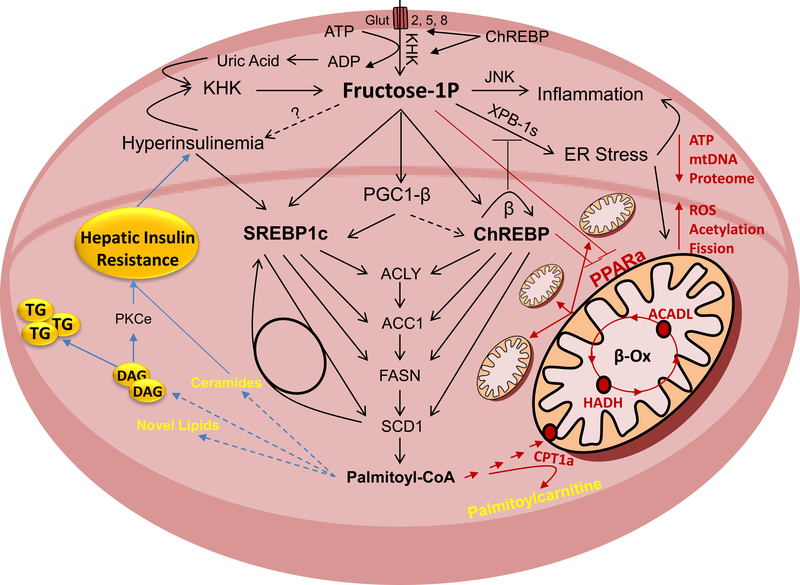
Fructose is metabolized in hepatocytes by ketohexokinase (KHK). This leads to decreased adenosine triphosphate (ATP) levels and increased uric acid production. Uric acid further stimulates KHK expression in a feed forward loop. Fructose strongly increases hepatic de novo lipogenesis (DNL). This is mediated through sterol regulatory element-binding protein 1c (SREBP1c) and carbohydrate-responsive element-binding protein (ChREBP) transcription factors that are at least in part regulated through peroxisome proliferator-activated receptor gamma coactivator 1-beta (PGC1b). They upregulate enzymes involved in free fatty acid (FFA) synthesis, such as ATP citrate lyase (ACLY), acetyl-CoA carboxylase 1 (ACC1), fatty acid synthase (FASN) and stearoyl-CoA desaturase 1 (SCD1). Accumulation of FFA in hepatocyte leads to insulin resistance, either directly or through buildup of more complex lipids and intermediates of lipid oxidation. In addition to DNL, fructose decreases mitochondrial fatty acid oxidation (FAO). This leads to a further decrease in ATP, decreased mitochondrial size and attenuated mitochondrial proteome, whereas mitochondrial fission, protein acetylation and reactive oxygen species (ROS) are increased. Decreased FAO is signaled through upregulation of ChREBP and a decrease in peroxisome proliferator activated receptor alpha (PPARα). Mitochondrial dysfunction and decreased FAO have been strongly linked with the development of hepatic insulin resistance. Fructose also strongly induces ER stress and hepatic inflammation, both of which can lead to hepatic insulin resistance.