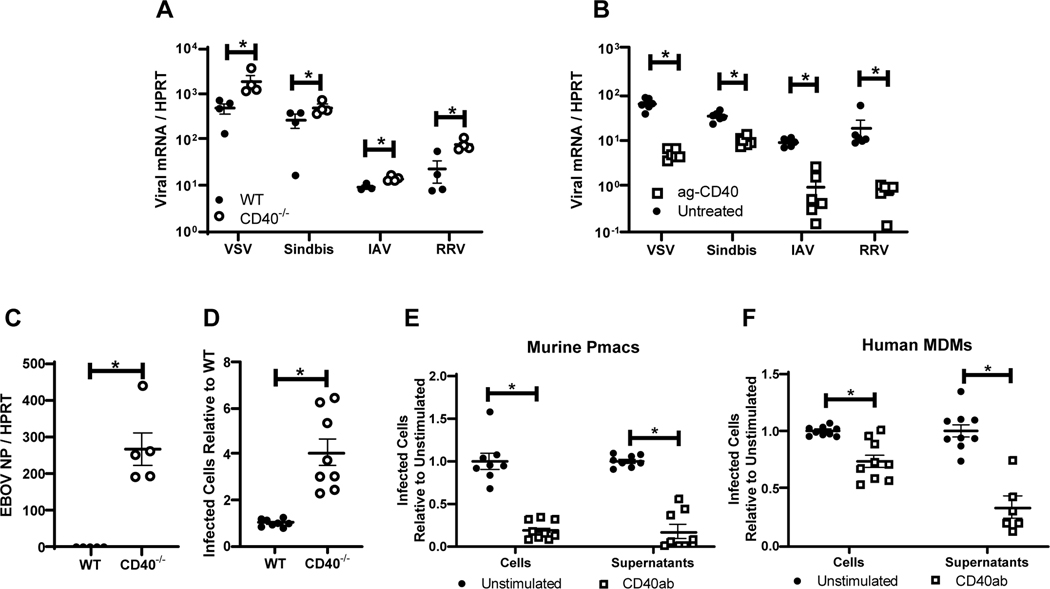
Figure 1. CD40 restricts replication of a variety of RNA viruses in cells from the peritoneal compartment.
A) Peritoneal cells from WT or CD40−/− male mice were infected with the indicated viruses (MOI=1 for all viruses except VSV where MOI = 0.5). Twenty-four hours following infection, RNA was extracted and viral load was quantified by qRT-PCR. B) Peritoneal cells from WT male mice were stimulated with an agonistic CD40 antibody, FGK4.5/FGK45, for 24 hours prior to infection and quantitation. C) Peritoneal cells from WT or CD40−/− mice were infected with EBOV (Mayinga) (MOI = 0.0015) and evaluated for virus load at 24 hours by qRT-PCR. D) Peritoneal cells from WT or CD40−/− mice were infected with by EBOV (Mayinga) (MOI = 0.0015) and number of infected cells were assessed at 24 hours by fixation followed by staining with anti-VP40 antibody and Hoescht dye. Infected cells were quantified by microscopy. E-F) Peritoneal cells from WT mice (E) or 6 day matured human MDMs (hMDMs) (F) were stimulated with an agonistic CD40 antibody (mouse CD40 antibody, FGK4.5/FGK45, or human CD40 antibody, G28.5) or left untreated (MOI = 0.0015 pmacs; MOI = 0.1 MDMs). After 24 hours, non-adherent cells were removed from the cultures and enriched pmacs or hMDMs were infected with EBOV(Mayinga). At 24 hours, cells were processed and stained as described in D. In parallel, supernatants from infected cells were titered on Vero cells and quantified using the same microscopic method. All experiments were performed at least three independent times. For all experiments, * indicates p<0.05. All qRT-PCR is quantified by delta Ct method comparing viral RNA to HPRT.