Abstract
Objectives:
Assessment of long-term outcome and toxicity of indigenous 177Lu-DOTATATE PRRT in patients of metastatic/advanced NETs in a large tertiary-care PRRT setting.
Methods:
A total of 468 metastatic/advanced NET patients (wide range of primary sites including CUP-NETs), who underwent at least two cycles of 177Lu-DOTATATE PRRT with available follow-up information, were included and analysed retrospectively in this study. In-house labelling of DOTATATE with 177Lu (direct route produced) was carried out in the hospital radiopharmacy and treatment administered in cycles (dose: 5.55 to 7.4 GBq per patient), at 10–12 weeks interval. The assessment of long-term outcome was undertaken under three broad headings: (a) Therapeutic response, (b) Survival outcome and (c) Toxicity assessment. The median point estimate with 95% CI for progression free survival (PFS) and overall survival (OS) were calculated by Kaplan–Meier method. Prognostic covariates for association with PFS and OS was investigated by Cox proportional hazards model (univariate and multivariate Hazard Ratios) and with disease control rate (DCR) by Chi-square test, with significant P value defined as <0.05.
Results:
Long-term outcome (follow-up ranging from 4 to 97.6 months; median period:46 months following first 177Lu-DOTATATE PRRT) results showed, (i) on symptomatic response evaluation scale, complete response (CR) in 214 patients (45.7%), partial response (PR) in 108 (23.1%), stable disease (SD) in 118 (25.2%), progressive disease (PD) in 28 (6%). (ii) Biochemical response evaluation showed CR in 52 (12%), PR in 172 (40%), SD in 161 (38%), and PD in 42 patients (10%). (iii) Molecular imaging response (by PERCIST criteria) showed CR in 29 (6%), PR in 116 (25%), SD in 267 (57%) and PD in 56 (12%) patients. (iv) On RECIST 1.1 criteria, CR was observed in 14 patients (3%), PR in 126 patients (27%), SD in 282 patients (60%) and PD in 46 patients (10%). The median PFS and OS were not reached at a median follow-up of 46 months. Observed PFS and OS at 7 years were 71.1% 95% CI (62.4–79.7%) and 79.4% 95% CI (71.4–86.9%) respectively. PFS was dependent on previous history of chemotherapy, baseline 68Ga-DOTATATE and 18F-FDG uptake, site of primary tumour, total cumulative dose and number of PRRT cycles on univariate analysis, whereas multivariate analysis showed significant association for previous history of chemotherapy, baseline 68Ga-DOTATATE and 18F-FDG uptake and number of PRRT cycles. The OS was dependent on baseline 68Ga-DOTATATE uptake, site of primary tumour, presence of bony metastatic disease, total cumulative dose and number of PRRT cycles on univariate analysis, whereas multivariate analysis showed significant association for bony metastatic disease and number of PRRT cycles. Transient haematological toxicity of Grade 1, Grade 2, and Grade 3 was found in 8 (1.7%), 1 (0.2%) and one patient (0.2%), respectively. Nephrotoxicity of Grade 1, Grade 2, Grade 3, and Grade 4 were seen in 16 (3.5%), 3 (0.6%), 2 (0.4%) and one patient (0.2%), respectively. On a separate sub-analysis of 322 NET patients with progressive disease at the initiation point of PRRT, overall response rates (CR + PR + SD) were 93.5%, 88.5%, 89.1 and 87.9% on symptomatic, biochemical, RECIST 1.1 and PERCIST criteria and PFS and OS at 7 years 68.3% and 79.2%, respectively.
Conclusions:
The present results demonstrate that 177Lu-DOTATATE PRRT improved symptoms and biochemical markers substantially in most of the NET patients, with disease stabilisation on both anatomical and molecular imaging in majority and response in a sizeable fraction. Additionally, the therapeutic protocol with lesser dose per cycle (mean 5.92 GBq/cycle) and prolonged duration (over 5 cycles and 1.5 years) in a metastatic NET setting proved equally efficacious (with superior PFS and OS rates) and relatively better tolerated with minimal toxicity.
Advances in knowledge:
The present work critically examines the long-term results, survival outcome and toxicity profile of the indigenous 177Lu-DOTATATE (produced through direct neutron activation of enriched 176Lu) in metastatic progressive NETs across a wide range of primary sites and malignancies. Such long-term outcome data establishes the favourable impact of PRRT in a wide patient base and also the therapeutic efficacy of the product.
Introduction
Neuroendocrine tumours (NETs) are relatively rare and heterogeneous neoplasms comprising of around 0.46% of gastrointestinal malignancies.1 They are most commonly located in the gastrointestinal system and pancreas.1–3 Surgery is the main curative treatment option but possible in limited number of NETs cases, since most cases present with advanced/metastatic disease at the time of diagnosis. Several other therapeutic options, for example, somatostatin analogues (SSA) therapy (as first-line medical treatment), target-directed therapies (as second-line treatment options), radiolabeled somatostatin analogues and chemotherapy are available for patients with advanced/metastatic NETs. The introduction of these new therapeutic options has resulted in better prognosis and progression-free survival (PFS). The unique characteristic of NET is over expression of somatostatin receptor (SSTR) on their tumour cells, which provides possibility of targeted theragnostic approach by employing radiolabeled somatostatin analogues such as 68Ga-DOTA-D-Phe-Tyr3-octreotate (68Ga-DOTATATE) for positron emission tomography-computed tomography (PET-CT) imaging and the β-emitter 177Lu-DOTA-D-Phe-Tyr3-octreotate (177Lu-DOTATATE) for peptide receptor radionuclide therapy (PRRT).4–10 The recently published randomised-controlled trial (NETTER-1) documented the clinical efficacy and safety profile of 177Lu-DOTATATE over long-acting octreotide in treating mid-gut NETs.11 However, the long-term survival and outcome results of 177Lu-DOTATATE PRRT in NETs continue to evolve and are being reported by the various large volume centres across the world.
The production of 177Lu in the nuclear reactor has two alternative strategies, that is, direct and indirect routes. In our centre for PRRT with 177Lu-DOTATATE, indigenously produced 177Lu was employed which originated following direct route production by irradiation of enriched lutetium target (82% 176Lu) at a thermal neutron flux of ~1.5 × 1014 n cm−2s.12 The present study explored the long-term therapeutic efficacy and toxicity data of this indigenous 177Lu-DOTATATE PRRT (InLuDOTA PRRT) in patients of metastatic /advanced NET in a large tertiary cancer care setting.
Methods and materials
A total of 512 metastatic/locally advanced NET patients were evaluated for PRRT in our institute from September 2010 till the time of analysis. Out of these patients, 468 patients who underwent at least two cycles of InLuDOTA PRRT were included and analysed retrospectively in this study. Patients who received 1 cycle of InLuDOTA PRRT or indirectly produced 177Lu and patients with less than 3-month follow-up after PRRT were excluded. This study was conducted in a single tertiary-care institute and was approved by the Institutional Scientific Advisory Committee (SAC) and Institutional Ethics Committee (IEC). The waiver for the informed consent was obtained in view of the retrospective nature of the study.
Inclusion and exclusion criteria for InLuDOTA PRRT
The eligibility criteria for InLuDOTA PRRT in our set-up included metastatic, advanced NET with or without progressive disease on other treatment modalities and a positive 68Ga-DOTATATE PET-CT (Krenning score ≥3, compared on MIP, coronal and transaxial images) pre-InLuDOTA PRRT.
The exclusion criteria for InLuDOTA PRRT included; negative 68Ga-DOTATATE PET-CT scan (Krenning score ≤2), NET patients with inadequate haematological, liver and renal function (haemoglobin <8 g dl−1, absolute neutrophil count <1500 mm−3, platelets < 75 × 109 L−1, bilirubin >1.5 × the upper normal limit and glomerular filtration rate <50 mL min−1 per 1.73 m2 body surface area), NET patients with Eastern Co-operative Oncology Group (ECOG) performance status 3–4 and Karnofsky Performance Status score of less than 30.
InLuDOTA PRRT therapy protocol
All 468 NET patients with metastatic/locally advanced disease had undergone the standard pre-PRRT work up protocol, which included imaging (68Ga-DOTATATE PET-CT and 18F-FDG PET-CT scans), documentation of clinical symptoms and measurement of biomarkers [serum chromogranin A (CgA) level] before administering InLuDOTA PRRT.
68Ga-DOTATATE PET-CT and 18F-FDG PET-CT scans
Approximately 60 min after intravenous injection of 74 to 111 MBq (2–3 mCi) of 68Ga-DOTATATE, whole body PET-CT scan was performed using time of flight (LYSO based PET crystal) PET-CT scanner. Whole body PET-CT scan was obtained after scout images in craniocaudal direction (voltage 120kVp, slice thickness 5 mm, pitch −0.83, FOV 600 mm rotation time-0.5second, 250 mA, image matrix 512 × 512) without any breath holding instructions. Contrast or non-contrast CT was used for diagnostic and attenuation correction of PET data. PET scanning was performed immediately after CT acquisition in the 3D emission mode with 3 min/bed position. Images were reconstructed iteratively using RAMLA algorithm with two iterations and 21 subsets. The acquired images were viewed (transaxial, sagittal and coronal views) in multimodality work station of Philips GEMINI TF processing system. Whole-body 18F-FDG PET-CT scan was performed 1 day after 68Ga-DOTATATE PET-CT scan maintaining the standard FDG-PET/CT imaging protocol.
Investigations and health-related quality of life scale assessment
The various investigations like complete blood counts (CBC), renal function tests (RFT), glomerular filtration rate (GFR), liver function test (LFT), serum CgA levels were estimated and documented in all NET patients before InLuDOTA PRRT. The patients were examined for health-related quality of life (HRQoL) scale assessment which included Eastern Cooperative Oncology Group (ECOG) Performance Status and Karnofsky score before and following InLuDOTA PRRT.
InLuDOTA PRRT administration
All NET patients were admitted in the radionuclide therapy ward for giving InLuDOTA PRRT. Short- and long-acting somatostatins analogues (SSA) were stopped for at least 24 h and 4 weeks before InLuDOTA PRRT administration, respectively.
In detail about production of 177Lu through direct route in Indian nuclear reactor was provided by elsewhere in the literature.12 A sterile solution of 177LuCl3 (direct route production) in 0.01M HCl with a specific activity of greater than 999 MBq/ug was supplied by Bhabha Atomic Research Centre (BARC), Mumbai, India. In-house 177Lu labelling of DOTATATE was carried in our institute with a radionuclide purity of greater than 99% for 177Lu-DOTATATE product. The detail of in-house labelling procedure of 177Lu with DOTATATE has been earlier provided by Das et al13 in their study. The product was approved by the Radiopharmaceutical Committee (RPC), the regulatory body of DAE (Department of Atomic Energy) India, for routine patient use.
All patients were examined clinically in radionuclide therapy ward before PRRT, which included general condition, blood pressure, pulse, respiratory rate and local abdominal examination. The patients received i.v. dexamethasone and ondensetron and one bottle (200 ml) of mixed amino acid infusion (containing positively charged lysine and arginine) before PRRT. Following this, 150–200 mCi (5.55 to 7.4 GBq) of InLuDOTA PRRT in 100 ml of normal saline was infused over 30 min. Further five more bottles of mixed amino acid infusion were administered over the next 7.5 h. All patients were monitored for 24 h after PRRT therapy for any acute adverse effects. InLuDOTA PRRT treatment was administered in cycles and cycles were repeated at intervals of 10–12 weeks.
Post-InLuDOTA PRRT scintigraphy
Whole-body planar imaging and regional single-photon emission CT (SPECT) imaging were conducted 24 h after PRRT using large field of view γ camera with medium energy collimator in all treated patients. Scan speed of 15 cm min−1 with matrix size 256×1024 and zoom of 1 was applied for whole-body imaging.
Post-InLuDOTA PRRT follow-up
All NET patients treated with PRRT were followed every 15 days for the following parameters that included complete blood count, RFT, LFT during course of PRRT cycles and at 6–12 months interval after the completion of PRRT. After every two cycles of PRRT, patients had undergone 68Ga-DOTATATE PET-CT and 18F-FDG PET-CT (if FDG positive lesions were present at baseline), regional anatomical imaging [MRI or contrast-enhanced CT (CeCT)], tumour markers (serum CgA level); also clinical and HRQoL scales evaluation was undertaken during the course of PRRT and at every 6–12 months after completion of PRRT. Any observed toxicity was recorded. Adverse events were graded according to the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v5.0) of the National Cancer Institute with special attention to nephrotoxicity, haematological toxicity and hepatotoxicity.
Long-term outcome of InLuDOTA PRRT
The long-term outcome of InLuDOTA PRRT was undertaken in three broad headings: (a) Therapeutic response, (b) Survival analysis and (c) Toxicity.
Therapeutic Response of InLuDOTA PRRT
Symptomatic Response: Subjective analogue scale and ECOG/Karnofsky scores
The symptomatic response was evaluated on the basis of an improvement in tumour-related symptoms based on the patient’s subjective report relative to baseline symptoms. The patients were asked at the follow-up with direct questioning on an analogue scale of 0 to 100% as to whether symptoms had “disappeared” or 90–100% improvement [complete response (CR)] or had “improved” 30 to 90% improvement (partial response [PR]) or were “stable disease” [<30% improvement/worsen; stable disease (SD)] or “worse” [≥30% increase in symptoms or new symptoms; progressive disease (PD)] compared with baseline. The baseline and follow-up ECOG/Karnofsky scores were also estimated in each patient.
Biochemical response
Serum CgA levels were measured during each follow-up period. Percentage change of serum CgA levels (tumour marker) was evaluated whether there was >75% decrease in tumour markers as CR, 30–75% decrease in values of tumour markers as PR, <30% decrease or increase in values of tumour markers as SD and ≥30% increase in values of tumour markers as PD.
Imaging response
Complete disappearance of abnormal uptake in previously avid lesions was defined as CR, ≥30% reduction in tracer uptake as PR, >30% increase in tracer uptake or appearance newly avid lesions as PD and neither PR nor PD as SD on 68Ga-DOTATATE and 18F-FDG PET-CT imaging according to the Hicks criteria as detailed in the PERCIST (PET Response Criteria in Solid Tumours) by Wahl et al.14,15
RECIST 1.1 (Response Evaluation Criteria in Solid Tumours) was employed16 for evaluation of anatomical imaging response following InLuDOTA PRRT with help of separate CeCT scan or diagnostic CT part of PET-CT. Based on RECIST 1.1 and PERCIST, patients post-InLuDOTA PRRT were categorized into responders (CR, PR, and SD = responders) and non-responders (PD). Disease control rate (DCR) was defined as CR +PR + SD on RECIST 1.1 criteria.
The survival analysis
Progression-free survival (PFS) was measured as the time between the date of first InLuDOTA PRRT and progression of disease on imaging
The overall survival (OS) was measured from the date of first InLuDOTA PRRT to the date of death from any cause or the date at last clinical follow-up.
Toxicity assessment
Acute toxicity including nausea and vomiting, hormonal crisis was documented in all patients during or after PRRT. Haematological toxicity, renal toxicity and hepatotoxicity were also evaluated as per the NCI-CTCAE v5.0.
Statistical analysis
All statistical analyses were performed using IBM SPSS software v.21. Discrete variables were summarised by counts (percentages) and continuous variables by their median (range), unless stated otherwise. The CR, PR, SD and PD in each of response evaluation categories (symptomatic, biochemical, PERCIST and RECIST 1.1) were calculated as aforementioned. The median point estimate with 95% CI for PFS and OS were calculated by the Kaplan–Meier method and rate of PFS and OS at 1 to 7 years were also calculated. Cox PH model was employed for analysing survival data. An unadjusted (univariate) and adjusted (multivariate) Hazard Ratio (HR) for various covariates were generated, along with 95% CI using the Cox PH model. Χ2 test was used to test association between different categorical types of variables. P value less than 0.05 considered to be statistically significant in all analyses.
Results
A total of 468 patients with locally advanced/metastatic NET(median age of 45 years, range 20–76 years, 289 males and 179 females), who had undergone at least two cycles of InLuDOTA PRRT in our institute from September 2010 to December 2019, were included and analysed retrospectively in this cohort study. Following primary tumour sites were noted: pancreas in 142 patients, small intestine in 112 patients, large intestine in 42 patients, stomach in 16 patients, gall bladder in nine patients, kidney in one patient, lung/mediastinum/thymus in 58 patients and unknown primary site (CUP-NETs) in 88 patients. The study cohort included WHO 2017 Grade 1 NET in 231 (49.3%) patients, Grade 2 in 207 (44.2%) patients and Grade 3 in 30 (6.5%) patients. Out of 468 patients, 162 (34.6%) patients had undergone resection of the primary before InLuDOTA PRRT, 161 patients received prior SSAs as the first-line treatment, 122 patients (26%) received prior chemotherapy (most of them received CAPTEM regimen comprising of capecitabine and temozolomide) and 40 patients (8%) received prior EBRT. Out of 468 patients in this study, 322 patients showed progressive disease on other therapeutic modalities before considering InLuDOTA PRRT. A total 400 patients (85.4%) were symptomatic with pain in abdomen as the most common complaint before InLuDOTA PRRT administration. Metastatic disease was found in 461 patients (98.5%) prior to InLuDOTA PRRT and remaining seven patients (1.5%) had inoperable locally advanced disease. Common metastatic sites in this cohort study included liver (n = 371 patients), lymph node (n = 127 patients) and skeleton (n = 85 patients). The total cumulative dose of InLuDOTA PRRT ranged from 12.76 GBq to 42.4 GBq per patient with average administered total cumulative dose of 30 GBq per patient and total number of InLuDOTA PRRT cycles ranged from 2 to 7 cycles per patient with average of 5 cycles per patient (average dose administered being 5.92 GBq per cycle) in this cohort. Long-term follow-up data were available and it ranged from 4 to 97.6 months with median follow-up duration of 46 months after the first InLuDOTA PRRT. The details of patient characteristics in the study population were demonstrated in Table 1.
Table 1.
Patients’ characteristics in the study population
| Patients characteristics | Value |
|---|---|
| Total no. of patients received at least 2 cycles of InLuDOTA PRRT | 468 |
| Sex (Males: Females) | 289:179 |
| Age: median, range in years | 45 years, 20–76 years |
| Symptomatic patients before InLuDOTA PRRT | 400 |
| Metastatic disease before InLuDOTA PRRT | 461 |
| Site of Primary Tumour | |
| Pancreatic | 142 |
| Small intestine | 112 |
| Large intestine | 42 |
| Stomach | 16 |
| Gall bladder | 9 |
| Kidney | 1 |
| Lung/mediastinum/thymus | 58 |
| Unknown primary site | 88 |
| Grade -WHO 2017 | |
| Grade 1 | 231 |
| Grade 2 | 207 |
| Grade 3 | 30 |
| Prior therapies | |
| Surgical resection of lesion | 162 |
| Chemotherapy | 122 |
| Somatostatin analogue therapy | 161 |
| External beam radiotherapy (EBRT) | 40 |
| Progressive disease prior to InLuDOTA PRRT | 322 |
| Doses and cycles of InLuDOTA PRRT patients received in this study | |
| Range of total cumulative dose of InLuDOTA PRRT patients received | 12.76 GBq to 42.4GBq |
| Average total cumulative dose of InLuDOTA PRRT patient received | 30 GBq |
| Range of total number of InLuDOTA PRRT cycles patient received | two to 7 cycles |
| Average number of InLuDOTA PRRT cycles patient received | five cycles |
Long-term outcome of InLuDOTA PRRT
Therapeutic response of InLuDOTA PRRT
Symptomatic response
Out of 468 patients, CR was found in 214 patients (45.7%), PR in 108 patients (23.1%), SD in 118 patients (25.2%), whereas PD was found in 28 patients (6%) on symptomatic response evaluation. Pre-InLuDOTA PRRT mean ECOG and KPS scores were 1.8 and 85, respectively, which changes to 1.9 and 86 following InLuDOTA PRRT.
Biochemical response
Tumour marker parameters were not available in 41 patients during follow-up period. Hence, biochemical response evaluation was possible in 427 patients out of 468 patients. CR was found in 52 patients (12%), PR in 172 patients (40%), SD in 161 patients (38%) and PD in 42 patients (10%) on biochemical response evaluation following InLuDOTA PRRT.
Imaging response
Out of the 468 patients, CR was found in 29 patients (6%), PR in 116 patients (25%), SD in 267 patients (57%) and PD in 56 patients (12%) on PERCIST criteria following InLuDOTA PRRT.
CR was observed in 14 patients (3%), PR in 126 patients (27%), SD in 282 patients (60%) and PD in 46 patients (10%) on RECIST 1.1 criteria after InLuDOTA PRRT as shown in Table 2.
Table 2.
Therapeutic Response of InLuDOTA PRRT in whole cohort of patients
| Response | Symptomatic response evaluation (No. of patients) | Biochemical response evaluation in 427 patients (No. of patients) | RECIST 1.1 (No. of patients) | PERCIST (No. of patients) |
|---|---|---|---|---|
| Complete Response | 214 (45.7%) | 52 (12%) | 14 (3%) | 29 (6%) |
| Partial Response | 108 (23.1%) | 172 (40%) | 126 (27%) | 116 (25%) |
| Stable Disease | 118 (25.2%) | 161 (38%) | 282 (60%) | 267 (57%) |
| Progressive Disease | 28 (6%) | 42 (10%) | 46 (10%) | 56 (12%) |
Survival
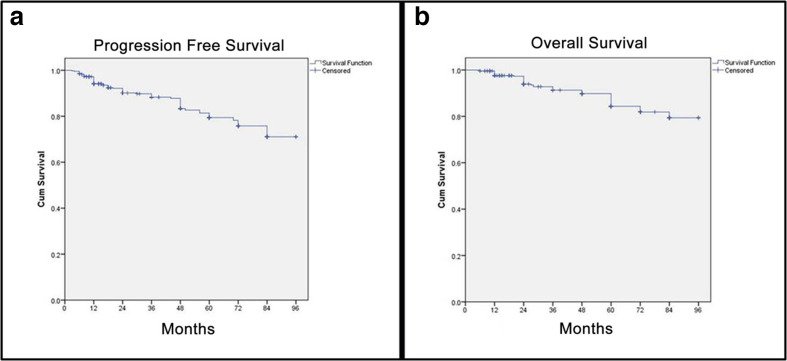
The median PFS and OS were not attained at a median follow-up of 46 months in the present study. The Kaplan-Meier curves for PFS and OS with estimated PFS rates of 71.1% 95 CI (62.4–79.7%) and OS rates of 79.4% 95 CI (71.4–86.9%) at 7 years have been depicted in Figure 1a and b, respectively.
Figure 1.
Kaplan-Meier curves for PFS and OS of NET patients after first InLuDOTA PRRT
Clinical toxicity
Acute toxicities of Grade 1 nausea were seen in 145 patients (30%). Grade 1 vomiting was seen in 58 patients (12%). Acute hormonal crisis was seen in one patient (0.2%) within 24 h after the administration of InLuDOTA PRRT.
Transient Grade 1, Grade 2 and Grade 3 haematological toxicity was found in eight patients (1.7%), one patient (0.2%) and one patient (0.2%), respectively. No Grade four haematological toxicity was found in any of the 468 patients during the follow-up period. Nephrotoxicity of Grade 1, Grade 2, Grade 3, and Grade 4 was found in 16 patients (3.5%), three patients (0.6%), two patients (0.4%) and one patient (0.2%), respectively. Hepatotoxicity of Grade 1 and Grade 2 was seen in one patient (0.2%) and one patient (0.2%) respectively as shown in Table 3.
Table 3.
Clinical toxicity
| Adverse events | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Nausea | 145 (30%) | 0 | 0 | 0 |
| Vomiting | 58 (12%) | 0 | 0 | 0 |
| Hematological toxicity | 8 (1.7%) | 1 (0.2%) | 1 (0.2%) | 0 |
| Nephrotoxicity | 16 (3.5%) | 3 (0.6%) | 2 (0.4%) | 1 (0.2%) |
| Hepatotoxicity | 1 (0.2%) | 1 (0.2%) | 0 | 0 |
On a separate sub-analysis of 322 NET patients (of the described 468), who had progressive disease at the initiation point of PRRT, the overall response rates (CR + PR+ SD) were 93.5%, 88.5%, 89.1% and 87.9% on symptomatic, biochemical, PERCIST and RECIST 1.1 criteria and PFS and OS at 7 years 68.3% and 79.2%, respectively (individual categorized results detailed in Table 4).
Table 4.
Therapeutic Response of InLuDOTA PRRT in 322 patients with progressive disease prior to InLuDOTA PRRT
| Response | Symptomatic response evaluation (No. of patients) | Biochemical response evaluation in 321 patients (No. of patients) | RECIST 1.1 (No. of patients) | PERCIST (No. of patients) |
|---|---|---|---|---|
| Complete Response | 138 (42.8%) | 43 (13.4%) | 13 (4%) | 23 (7.1%) |
| Partial Response | 72 (22.4%) | 127 (39.6%) | 86 (26.7%) | 83 (25.8%) |
| Stable Disease | 91 (28.3%) | 114 (35.5%) | 188 (58.4%) | 177 (55%) |
| Progressive Disease | 21 (6.5%) | 37 (11.5%) | 35 (10.9%) | 39 (12.1%) |
Significant association of PFS, OS and DCR with various covariates
PFS
Significant association of PFS with various variables was determined in terms of HR (95% CI) and p value. Significant p value was obtained for following variables: (a) prior history of chemotherapy [Patient who did not receive prior chemotherapy had lower risk with HR = 0.59 95% CI (0.36–0.97) compared to those who received prior chemotherapy; p = 0.037], (b) baseline 18F-FDG uptake in lesions [SUVmax ≥5 was found increased risk with HR = 2.18 95% CI (1.35–3.53) compared to uptake of SUVmax <5; p < 0.05], (c) baseline 68Ga-DOTATATE uptake in lesions [SUVmax <20 was reported high risk with HR = 2.19 95% CI (1.35–3.56), compared to uptake of SUVmax ≥20; p < 0.05], (d) for primary sites, HR was significantly higher for mediastinal NET [HR = 4.66 (95% CI (1.47–14.7), p < 0.05] in reference to small intestine, (e) total cumulative dose of InLuDOTA PRRT [Patients who received total cumulative dose of InLuDOTA PRRT < 29.6 GBq reported higher risk with HR = 3.89 95% CI (2.14–7.08) compared to patient who received dose of ≥29.6 GBq; p < 0.05], and (f) number of InLuDOTA PRRT cycles (more risk with HR = 5.62 95% CI (3.14–10.07) was estimated for those received less than 5 InLuDOTA PRRT cycles compared to ≥5 cycles; p < 0.05}. Multivariate analysis was done and all above-mentioned variables were found significantly high HR except total cumulative dose of InLuDOTA PRRT the patient received (<29.6 GBq; p = 0.7). We have to mention here that multivariate analysis could not be performed for primary sites due to insufficient number for each primary site as shown in Table 5.
Table 5.
Association of PFS, OS and DCR with various variables
| PFS | ||||
|---|---|---|---|---|
| Prognostic Variables | HR (95% CI) | p value | Adjusted HR (95% CI) | p value |
| Previous Chemotherapy | ||||
| Yes | 1.0 | 1.0 | ||
| No | 0.59 (0.36–0.97) | 0.037 | 0.60 (0.36–0.98) | 0.04 |
| Baseline FDG uptake | ||||
| SUVmax <5 | 1.0 | 1.0 | ||
| SUVmax ≥5 | 2.18 (1.35–3.53) | <0.05 | 1.91 (1.16–3.12) | 0.01 |
| Baseline 68Ga DOTATATE uptake | ||||
| SUVmax ≥20 | 1.0 | 1.0 | ||
| SUVmax <20 | 2.19 (1.35–3.56) | <0.05 | 1.63 (1.0–2.68) | 0.05 |
| Site of primary | ||||
| Small intestinal NET | Not performed due to insufficient number | |||
| Large intestine | 1.0 (0.03–1.54) | 0.12 | ||
| Pancreas | 1.24 (0.58–2.65) | 0.57 | ||
| Lung | 1.44 (0.50–4.16) | 0.49 | ||
| Mediastinal | 4.66 (1.47–14.7) | <0.05 | ||
| Total cumulative dose of InLuDOTA PRRT | ||||
| ≥29.6 GBq | 1.0 | 1.0 | ||
| <29.6 GBq | 3.89 (2.14–7.08) | <0.05 | 1.21 (0.44–3.32) | 0.7 |
| No. of InLuDOTA PRRT cycles | ||||
| ≥5 cycles | 1.0 | 1.0 | ||
| <5 cycles | 5.62 (3.14–10.07) | <0.05 | 4.11 (1.55–10.91) | <0.05 |
| OS | ||||
| Prognostic Variables | HR (95% CI) | p value | Adjusted HR | p value |
| Baseline 68Ga-DOTATATE uptake | ||||
| SUVmax ≥20 | 1.0 | 1.0 | ||
| SUVmax <20 | 2.46 (1.36–4.44) | <0.05 | 1.31 (0.71–2.44) | 0.38 |
| Presence of bony metastases | ||||
| No | 1.0 | 1.0 | ||
| Yes | 2.13 (1.14–3.95) | 0.017 | 2.13 (1.13–4.01) | 0.019 |
| Site of primary | ||||
| Small intestinal NET | 1.0 | Not performed due to insufficient number | ||
| Large intestine | - | |||
| Pancreas | 1.03 (0.38–2.77) | 0.95 | ||
| Lung | 2.27 (0.72–7.15) | 0.16 | ||
| Mediastinal | 8.70 (2.75–27.5) | <0.05 | ||
| Total cumulative dose of InLuDOTA PRRT | ||||
| ≥29.6 GBq | 1.0 | 1.0 | ||
| <29.6 GBq | 6.84 (2.87–16.26) | <0.05 | 0.83 (0.18–3.68) | 0.80 |
| No. of InLuDOTA PRRT cycles | ||||
| ≥5 cycles | 1.0 | 1.0 | ||
| <5 cycles | 11.77 (4.91–28.22) | <0.05 | 12.90 (2.85–58.40) | <0.05 |
| p value for DCR | ||||
| Yes | No | p values | ||
| Baseline FDG uptake | ||||
| SUVmax <5 | 279 (93.3%) | 20 (6.7%) | ||
| SUVmax ≥5 | 143 (84.6%) | 26 (15.4%) | 0.02 | |
| 68Ga-DOTATATE uptake | ||||
| SUVmax ≥20 | 311 (92.8%) | 24 (7.2%) | ||
| SUVmax <20 | 111 (83.5%) | 22 (16.5%) | 0.002 | |
| Site of primary | ||||
| Small intestinal NET | 107 (93%) | 8 (7%) | ||
| Large intestine | 43 (100%) | 0 (0%) | ||
| Pancreas | 131 (91%) | 13 (9%) | ||
| Lung | 31 (88.6%) | 4 (11.4%) | ||
| Mediastinal | 7 (63.6%) | 4 (36.4%) | 0.05 | |
| Bony metastases | ||||
| Yes | 71 (83.5%) | 14 (16.5%) | ||
| No | 351 (91.6%) | 32 (8.4%) | 0.023 | |
| Baseline serum CgA level | ||||
| <400 ng ml−1 | 177 (95.7%) | 8 (4.3%) | ||
| ≥400 ng ml−1 | `227 (87%) | 34 (13%) | <0.05 | |
| Total cumulative dose of InLuDOTA PRRT | ||||
| ≥29.6 GBq | 175 (95.1%) | 9 (4.9%) | ||
| <29.6 GBq | 247 (87%) | 37 (13%) | <0.05 | |
| No of InLuDOTA PRRT cycles | ||||
| ≥5 cycles | 217 (96%) | 9 (4%) | ||
| <5 cycles | 205 (84.7%) | 37 (15.3%) | <0.05 | |
OS
Significant association of OS with various variables was determined in terms of HR (95% CI) and p value. Significant P value was found for following variables: (a) baseline 68Ga-DOTATATE uptake in lesions [SUVmax <20 reported high risk HR = 2.46 95% CI (1.36–4.44) compared to uptake of SUVmax ≥20; p < 0.05], (b) site of primary disease [HR was significantly higher for mediastinal NET with HR of 8.70 95% CI (2.75–27.5) in reference to small intestine NET; p < 0.05], (c) presence of bony metastatic disease [patients with bony metastases reported high risk with HR = 2.13 95% CI (1.14–3.95) compared to those without bony metastases; p = 0.017], (d) total cumulative dose of InLuDOTA PRRT patient received [patient who received <29.6 GBq total cumulative dose of InLuDOTA PRRT reported high risk with HR of 6.84 95% CI (2.87–16.26) compared to patient who received dose of ≥29.6 GBq; p < 0.05], and (e) number of InLuDOTA PRRT cycles [more risk with HR = 11.77 95% CI (4.91–28.22) was estimated for those received less than five cycles of InLuDOTA PRRT compared to ≥5 cycles; p < 0.05]. On multivariate analysis, presence of bony metastases and less than five cycles of InLuDOTA PRRT was found to be significantly high, with HR of 2.13 [95% CI (1.13–4.01), p = 0.019] and 12.90 [95% CI (2.85–58.40), p < 0.05], respectively. We could not perform multivariate analysis for primary site due insufficient number for each primary site as depicted in Table 5.
DCR
Significant P value for DCR, in terms of better control rate was documented for following variables: (a) baseline 18F-FDG uptake in lesions (SUVmax <5), (b) baseline 68Ga-DOTATATE uptake in lesions (SUVmax ≥20), (c) site of primary disease (small intestine NET), (d) absence of bony metastatic disease, e) baseline serum CgA level (<400 ng ml−1) f) total cumulative dose of InLuDOTA PRRT patient received (≥29.6 GBq), and g) number of InLuDOTA PRRT cycles (≥5 cycles) as illustrated in Table 5.
Discussion
In a retrospective analysis of 64,791 patients of NET by Surveillance, Epidemiology, and End Results (SEER) program for the period 1973–2012, to determine 5 years OS, the median OS rate was 9.3 years (112 months) while for patients with distant metastatic disease it was only 12 months.17 In another study using SEER database, analyzing 73,782 NET patients the median OS was 41 months and 1 year, 3 years, 5 years and 10 years OS of 72.8, 52.7, 39.4 and 18.1%, respectively.18 These two databases directly provided evidence of lower OS in NET patients with metastatic disease.
In the peer-reviewed literature, a few studies have provided long-term outcome of 177Lu-DOTATATE PRRT and improvement in survival after PRRT. Ezzidin et al19 retrospectively analysed 74 metastatic gastroenteropancreatic neuroendocrine (GEP-NET) patients and provided long-term outcome of 177Lu-DOTATATE PRRT; post-PRRT response was calculated by using modified Southwest Oncology Group criteria and they found PR of 36.5%, minor response (MR) of 17.6%, SD of 35.1%, and PD of 10.8%. The median PFS and OS were 26 months 95% CI (18.3–33.7) and 55 months 95% CI (48.8–61.2), respectively. They also found Ki-67 index, performance status, tumour burden and neuron-specific enolase as independent predictors of survival. In comparison, the present study explored a substantially larger number of metastatic NET patients and with additionally availability of results with molecular based imaging and RECIST based response evaluation.
In another study performed by Brabander et al20, a group of 443 NET patients was analysed for efficacy and survival following 177Lu-DOTATATE therapy. They found an objective response rate (ORR) of 39% and SD of 43%on RECIST 1.1, with PFS of 29 months [95% CI (26–33) months] and OS of 63 months [95% CI (55–72) months]. The long-term toxicity included acute leukaemia in four patients (0.7%) and myelodysplastic syndrome (MDS) in nine patients (1.5%). The main limitations of their study were non-randomised and retrospective study design and included a heterogeneous group of NET patients, and non-availability of association data of PFS and OS with various variables.
Baum et al21 analysed a total of 1048 NET patients following PRRT. Out of 1048 patients, 378 NET patients received 177Lu-DOTATATE therapy (as a monotherapy), 513 patients received 177Lu-DOTATATE in combination with 90Yttrium either as a combination of both radionuclides in one cycle (TANDEM) or sequentially (DUO) and remaining 157 patients received 90Y-DOTATATE or-DOTATOC as monotherapy in their study. Out of 1048 patients, 573 (54.7%) were alive at the end of the study with median OS of 51 months. They found the median PFS of 19 months after first PRRT. In their study, PFS was found to be influenced by type of radionuclide used, grading and site of primary, whereas OS was found to depend upon the type of radionuclide, previous therapy and site of primary tumour. They concluded that PRRT in NETs significantly improved OS and PFS with minimum adverse effects.
Aalsbersberg et al22 studied the clinical and treatment parameters associated with PFS and OS. They included 782 NET patients treated with 177Lu (37.7%), 90Y (12.3%) or both (50%) radionuclides with a median follow-up period of 42.7 months. They found median PFS of 22 months and OS of 53 months. In their study, PFS was dependent upon Ki-67 index, previous interferon therapy and chemotherapy, presence of diabetes and serum CgA level. OS was dependent upon Ki-67, performance status, previous chemotherapy, radiotherapy ablation and serum CgA level.
In our study, following InLuDOTA PRRT, the observed CR and PR were 45.7 and 23.1% on symptomatic response, 12 and 40% on biochemical response, 3 and 27% on RECIST 1.1 and 6 and 25% on PERCIST criteria, respectively. Disease progression was observed in 10% on RECIST 1.1 and 12% on PERCIST criteria following InLuDOTA PRRT treatment. Ezzidin et al and Brabander et al found ORR of 36.5 and 39% after PRRT in their study, respectively. The observed response rate of 31 and 30% on PERCIST and RECIST 1.1 criteria was seen in our study, respectively, which was similar to the earlier mentioned reports.
The median PFS and OS were not reached at the time of analysis in our study. In the previously mentioned studies, maximum median PFS and OS was reported by Brabander et al, which were 29 months and 63 months, respectively, in a population of 443 patients. In our study, in a total of 468 NET patients,PFS and OS estimated rates at 7 years were 71.1% 95% CI (62.4–79.7%) and 79.4% 95% CI (71.4–86.9%), respectively. On a separate analysis of 322 NET patients (of described 468) with progressive disease at the initiation point of PRRT, overall response rates (CR + PR + SD) were 93.5%, 88.5%, 89.1% and 87.9% on symptomatic, biochemical, RECIST 1.1 and PERCIST criteria and PFS and OS at 7 years 68.3% and 79.2%, respectively. In comparison with previously mentioned studies, the present study reported long-term outcome in larger patient cohort who had undergone 177Lu-DOTATATE monotherapy and registered gratifying PFS and OS results.
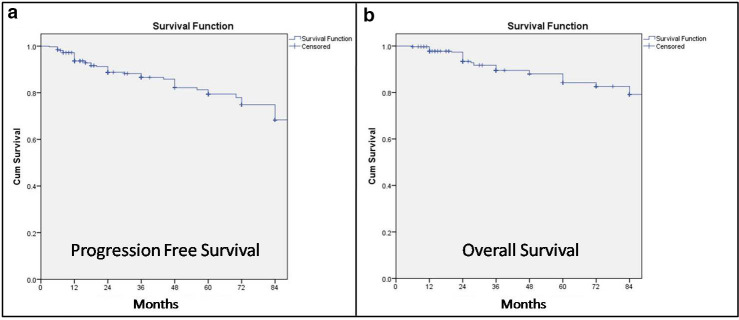
With respect to prognostic variables for PFS and OS (analysed by both Baum et al and Aalsberger et al in their studies), we observed similar variables as reported by other investigators. In the present study, the PFS was dependent on previous history of chemotherapy, baseline 68Ga-DOTATATE and 18F-FDG uptake in lesions, site of primary tumour, total cumulative dose and number of InLuDOTA PRRT cycles on univariate analysis, whereas multivariate analysis showed significant association for previous history of chemotherapy, baseline 68Ga-DOTATATE and 18F-FDG uptake in lesions and number of InLuDOTA PRRT cycles. The OS was dependent on baseline 68Ga-DOTATATE uptake in lesions, site of primary tumour, presence of bony metastatic disease, total cumulative dose and number of InLuDOTA PRRT cycles on univariate analysis, whereas multivariate analysis showed significant association for bony metastatic disease and number of InLuDOTA PRRT cycles. The present study also analysed DCR, which was dependent on baseline 68Ga-DOTATATE and 18F-FDG uptake in lesions, site of primary tumour, bony metastatic disease, baseline serum CgA level, total cumulative dose and number of InLuDOTA PRRT cycles. In a separate sub analysis amongst the 322 patients, who had progressive disease at time of initiation of InLuDOTA PRRT, similar therapeutic response and survival rates were documented following InLuDOTA PRRT as depicted in Table 4 and Figure 2a and b.
Figure 2.
Kaplan-Meier curves for PFS and OS after first InLuDOTA PRRT in NET patients (presenting with progressive disease)
In our study, transient haematological toxicity of Grade 1, Grade 2 and Grade 3 was noted in eight patients (1.7%), one patient (0.2%) and one patient (0.2%), respectively. No Grade 4 haematological toxicity was found in any of the 468 patients. Nephrotoxicity of Grade 1, Grade 2, Grade 3 and Grade 4 were seen in 16 patients (3.5%), three patients (0.6%), two patients (0.4%) and one patient (0.2%), respectively. Baum et al and Brabander et al found MDS/leukemia of 2.1% and 2.2% in their studies respectively. In our study, MDS/leukemia was not seen at time analysis of this data.
In the present study, indigenously produced direct (n,γ) route 177Lu was employed (which is used in most centres in Indian centres primarily due to its substantially low cost), which involves a number of advantages over the indirect route produced 177Lu (commonly used in PRRT with imported 177Lu preparation): (i) at the production level, the simple post-irradiation chemical treatment procedure in direct route makes production of 177Lu less technologically demanding and (ii) at the user level, much more cost-effective compared to indirect-route produced 177Lu. Data on long-term outcome of 177Lu-DOTATATE monotherapy (from direct route), especially PFS and OS are lacking at present in the peer-reviewed literature. In our study, favourable long-term outcome of direct route produced 177Lu (as InLuDOTA PRRT) was observed in a large number of metastatic advanced NET patients, establishing the long-term efficacy of the product.
Furthermore, we believe that even within the context of fixed dose regimen, the therapeutic schedule and protocol of PRRT could be in a way individualised based upon the clinical scenario and intent of therapy in a patient.23 In our setting, the patients with multiple metastatic advanced disease was treated with average total cumulative 177Lu-DOTATATE dose of 29.6 GBq per patient over five cycles and 1.5 years (mean 5.92 GBq/cycle).12,23 This was in contrast to those treated for neoadjuvant purpose where standard 7.4 GBq was administered strictly at eight weekly interval. This prolonged duration of PRRT cycles over 1.5 to 2 years, in metastatic palliative setting, with lesser 177Lu-DOTATATE dose per cycle might have resulted better PFS and OS and low incidence of side-effects in our study as compared to other reported studies. The study, however, was limited by its retrospective nature which may introduce bias, however, we used uniform InLuDOTA PRRT protocol in this entire cohort study as per the standard Institutional standardised operating procedure, which had fixed dose regimen, administration and uniform follow-up protocol for all patients.
Conclusion
The present results demonstrated that 177Lu-DOTATATE PRRT improved the symptoms and biochemical markers substantially in most of the metastatic NET patients, with disease stabilisation on both anatomical and molecular imaging in majority and also improvement in a sizeable fraction of patients., The results indicated that indigenous 177Lu-DOTATATE PRRT (obtained through direct neutron activation route) was equally efficacious in addition to its obvious cost-effectiveness. Longer PFS was dependent upon FDG and SSTR uptake in lesions, no previous history of chemotherapy, and number of PRRT cycles on both univariate and multivariate analysis and site of primary tumour, total cumulative dose of InLuDOTA on univariate analysis. Longer OS was dependent upon absence of bony metastases and number of PRRT cycles on both univariate and multivariate analysis and SSTR uptake in lesions, site of primary tumour, and total cumulative dose of InLuDOTA on univariate analysis. Additionally, the therapeutic protocol with lesser dose per cycle (mean 5.92 GBq/cycle) and prolonged duration (over five cycles and 1.5 years) in metastatic NET setting proved equally efficacious (with superior PFS and OS rates) and relatively better tolerated with minimal toxicity.
Contributor Information
Keerti Sitani, Email: keerti.sitani@gmail.com.
Rahul V Parghane, Email: rahul_parghane@yahoo.co.in.
Sanjay Talole, Email: sdtalole@gmail.com.
Sandip Basu, Email: drsanb@yahoo.com.
REFERENCES
- 1.Niederle B, Pape U-F, Costa F, Gross D, Kelestimur F, Knigge U, et al. . ENETS consensus guidelines update for neuroendocrine neoplasms of the jejunum and ileum. Neuroendocrinology 2016; 103: 125–38. doi: 10.1159/000443170 [DOI] [PubMed] [Google Scholar]
- 2.Hallet J, Law CHL, Cukier M, Saskin R, Liu N, Singh S, et al. . Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015; 121: 589–97. doi: 10.1002/cncr.29099 [DOI] [PubMed] [Google Scholar]
- 3.Fraenkel M, Kim MK, Faggiano A, Valk GD. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol 2012; 26: 691–703. doi: 10.1016/j.bpg.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 4.Modlin IM, Moss SF, Chung DC, Jensen RT, Snyderwine E. Priorities for improving the management of gastroenteropancreatic neuroendocrine tumors. J Natl Cancer Inst 2008; 100: 1282–9. doi: 10.1093/jnci/djn275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modlin IM, Champaneria MC, Chan AKC, Kidd M. A three-decade analysis of 3,911 small intestinal neuroendocrine tumors: the rapid pace of NO progress. Am J Gastroenterol 2007; 102: 1464–73. doi: 10.1111/j.1572-0241.2007.01185.x [DOI] [PubMed] [Google Scholar]
- 6.Sato Y, Hashimoto S, Mizuno K-I, Takeuchi M, Terai S. Management of gastric and duodenal neuroendocrine tumors. World J Gastroenterol 2016; 22: 6817–28. doi: 10.3748/wjg.v22.i30.6817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yordanova A, Mayer K, Brossart P, Gonzalez-Carmona MA, Strassburg CP, Essler M, et al. . Safety of multiple repeated cycles of 177Lu-octreotate in patients with recurrent neuroendocrine tumour. Eur J Nucl Med Mol Imaging 2017; 44: 1207–14. doi: 10.1007/s00259-017-3652-1 [DOI] [PubMed] [Google Scholar]
- 8.Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, de Herder WW, Feelders RA, et al. . Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol 2005; 23: 2754–62. doi: 10.1200/JCO.2005.08.066 [DOI] [PubMed] [Google Scholar]
- 9.Geijer H, Breimer LH. Somatostatin receptor PET/CT in neuroendocrine tumours: update on systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2013; 40: 1770–80. doi: 10.1007/s00259-013-2482-z [DOI] [PubMed] [Google Scholar]
- 10.Sharma P, Singh H, Bal C, Kumar R. PET/CT imaging of neuroendocrine tumors with (68)Gallium-labeled somatostatin analogues: An overview and single institutional experience from India. Indian J Nucl Med 2014; 29: 2–12. doi: 10.4103/0972-3919.125760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. . Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 2017; 376: 125–35. doi: 10.1056/NEJMoa1607427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basu S, Parghane RV, Kamaldeep CS, Chakrabarty S. Peptide receptor radionuclide therapy of neuroendocrine tumors. Semin Nucl Med 2020; 50: 447–64. doi: 10.1053/j.semnuclmed.2020.05.004 [DOI] [PubMed] [Google Scholar]
- 13.Das T, Chakraborty S, Kallur KG, Venkatesh M, Banerjee S, al at. Preparation of patient doses of (177)Lu-DOTA-TATE using indigenously produced (177)Lu: the Indian experience. Cancer Biother Radiopharm 2011; 26: 395–400. doi: 10.1089/cbr.2010.0881 [DOI] [PubMed] [Google Scholar]
- 14.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009; 50 Suppl 1: 122S–50. doi: 10.2967/jnumed.108.057307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parghane RV, Talole S, Prabhash K, Basu S. Clinical response profile of metastatic/advanced pulmonary neuroendocrine tumors to peptide receptor radionuclide therapy with 177Lu-DOTATATE. Clin Nucl Med 2017; 42: 428–35. doi: 10.1097/RLU.0000000000001639 [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 17.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. . Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017; 3: 1335–42. doi: 10.1001/jamaoncol.2017.0589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Man D, Wu J, Shen Z, Zhu X. Prognosis of patients with neuroendocrine tumor: a SEER database analysis. Cancer Manag Res 2018; 10: 5629–38. doi: 10.2147/CMAR.S174907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ezziddin S, Attassi M, Yong-Hing CJ, Ahmadzadehfar H, Willinek W, Grünwald F, et al. . Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med 2014; 55: 183–90. doi: 10.2967/jnumed.113.125336 [DOI] [PubMed] [Google Scholar]
- 20.Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW, et al. . Long-term Efficacy, survival, and safety of [177Lu-DOTA0,Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res 2017; 23: 4617–24. doi: 10.1158/1078-0432.CCR-16-2743 [DOI] [PubMed] [Google Scholar]
- 21.Baum RP, Kulkarni HR, Singh A, Kaemmerer D, Mueller D, Prasad V, et al. . Results and adverse events of personalized peptide receptor radionuclide therapy with 90Yttrium and 177Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget 2018; 9: 16932–50. doi: 10.18632/oncotarget.24524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aalbersberg EA, Huizing DMV, Walraven I, de Wit-van der Veen BJ, Kulkarni HR, Singh A, et al. . Parameters to predict progression-free and overall survival after peptide receptor radionuclide therapy: a multivariate analysis in 782 patients. J Nucl Med 2019; 60: 1259–65. doi: 10.2967/jnumed.118.224386 [DOI] [PubMed] [Google Scholar]
- 23.Basu S, Chakraborty S, Parghane RV, Ranade R, Thapa P, et al. . One decade of 'Bench-to-Bedside' peptide receptor radionuclide therapy with indigenous [177Lu]Lu-DOTATATE obtained through 'Direct' neutron activation route: lessons learnt including practice evolution in an Indian setting. Am J Nucl Med Mol Imaging 2020; 10: 178–211. [PMC free article] [PubMed] [Google Scholar]