Figure 4.
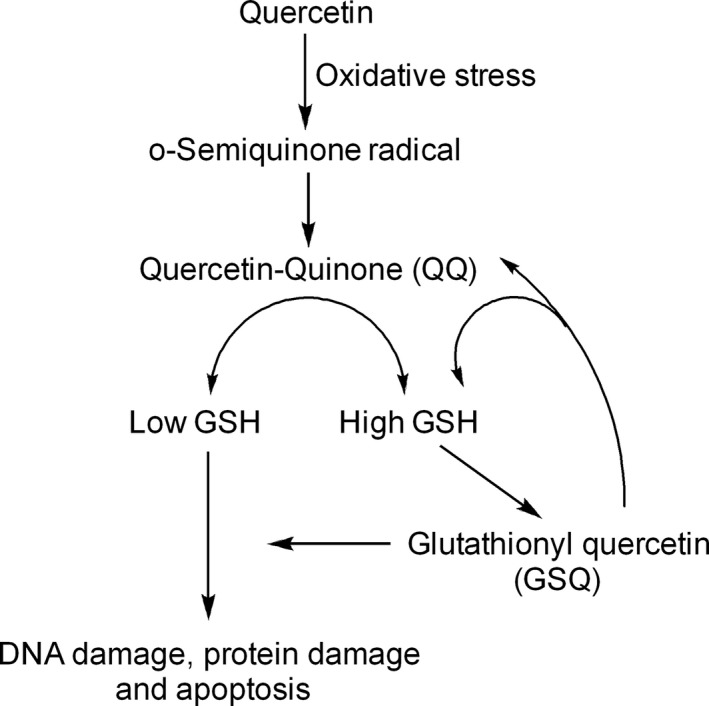
The molecular mechanism of switching between pro‐oxidant and antioxidant effects of quercetin. The switching between pro‐oxidant and antioxidant effects of quercetin is decided by its interaction with reduced glutathione (GSH). During oxidative stress, quercetin reacts with hydrogen peroxide (H2O2) in the presence of enzyme peroxidase to form semoquinone‐radicals which are immediately oxidized to form quercetin–quinone product (QQ). QQ products strong are strong pro‐oxidant and proapoptic moieties that react with protein thiols and DNA to induce apoptosis. Upon reaction with GSH,QQ form glutathionylquercetin (GSQ) adducts such as 8‐GSQ and 6‐GSQ. Interestingly, this reaction is reversible and allows dissociation of GSQ into GSH and QQ. In the presence of high GSH levels, QQ reacts with GSH to form GSQ and QQ again and in this situation QQ does not accumulate enough to induce apoptosis. However, in the presence of low levels of GSH,QQ accumulates and reacts with protein thiols and DNA to induce apoptosis.
