Abstract
Introduction:
Biomarkers of inflammation in blood and sputum can play a critical role in anti-inflammatory drug development in cystic fibrosis (CF). The objectives of this analysis were to examine relationships between airway and systemic measurements of inflammation, associations between inflammatory biomarkers and FEV1, differences in airway and systemic inflammation by baseline covariates, reproducibility of serum biomarkers, and to assess the effects of freezing and delayed processing on sputum analyte measurements.
Methods:
We analyzed baseline and serial concentrations of inflammatory markers in blood and induced sputum collected from individuals with CF ages 10 years and older who participated in a multicenter clinical trial.
Results:
Among circulating biomarkers, serum high sensitivity C-reactive protein (hsCRP) and serum amyloid A (SAA) correlated most strongly with each other (rs = 0.85). Comparing sputum-based inflammation measurements, sputum neutrophil elastase and myeloperoxidase (MPO) were the most highly correlated (rs = 0.88). Markers most strongly correlated with ppFEV1 were serum hsCRP (rs = −0.55), SAA (rs =−0.58), and sputum neutrophil elastase (rs = −0.53). Within-subject standard deviation was consistently lower than between-subject standard deviation for all serum biomarkers. Serum calprotectin and MPO had the highest ratio of between-to-within subject variability. Freezing and delayed sputum processing were not associated with significant differences in measurements of sputum neutrophil elastase, IL-1β, or MPO.
Conclusions:
Among the biomarkers analyzed, serum hsCRP and sputum neutrophil elastase are promising candidates to include in CF anti-inflammatory clinical trials to avoid redundancy, minimize variation, and serve as correlates of lung disease severity and change.
Keywords: cystic fibrosis, inflammation, biomarkers, lung function, sputum
1.0. INTRODUCTION
Neutrophil-dominated airway inflammation is a hallmark feature of cystic fibrosis (CF) lung disease, playing a key role in its pathogenesis and progression (1). Consequently, a major emphasis has been placed on evaluating drugs that target inflammation. Corticosteroids and high-dose ibuprofen, two of the first anti-inflammatory drugs studied in CF, both demonstrated clinical benefit (2–4), but side effects and other considerations have limited their use (5–7). However, evidence that these anti-inflammatory agents can slow the progression of CF lung disease, suggests that strategies to modulate lung inflammation can be beneficial. Additionally, limited data showing that ivacaftor does not dramatically impact airway inflammation over 6 months signifies that anti-inflammatory treatments are still needed with emerging highly effective CF transmembrane conductance regulator (CFTR) modulator therapies (8).
A major challenge confronting the development of new anti-inflammatory drugs is that standard clinical outcome measures often included in phase II trials such as lung function and pulmonary exacerbations, may not significantly change during the typical length of a phase II study. A CF Foundation working group convened to develop a strategic plan for anti-inflammatory drug development recommended that sputum and serum biomarkers of inflammation be included in anti-inflammatory clinical trials (9). Biomarkers of inflammation have the potential to screen anti-inflammatory candidates for biologic activity over a shorter time period, establish proof-of-concept, and help inform early go/no-go decisions.
Critical gaps in knowledge must be addressed to confidently rely upon sputum and circulating biomarkers as outcome measures in CF clinical trials. To reduce redundancy and standardize the selection of biomarkers as study endpoints, we need to better understand the inter-relationship between airway and systemic inflammation. Few studies have looked at sputum and blood-based inflammatory measurements simultaneously (10–12). While there are limited data correlating airway and systemic inflammation with percent predicted forced expiratory volume in one second (ppFEV1) in CF, derived mainly from single center studies (13–15), we must further determine associations between inflammatory biomarkers and ppFEV1 in broader CF populations. In addition, investigating differences in inflammation among children and adults with CF and between those with impaired and preserved lung function may help with subject selection for clinical trials, enriching trials with individuals who might experience a larger change in inflammation in response to a beneficial treatment. Furthermore, investigators and pharmaceutical companies often ask whether sputum needs to be immediately processed (i.e. homogenized) following collection or whether it can be frozen following collection and shipped to a centralized laboratory for processing. To inform multicenter trials that rely upon sputum outcome measures, we need to study the effects of freezing and delayed processing on sputum analyte measurements.
The primary objectives of this study were to examine relationships between airway and systemic measurements of inflammation, associations between sputum and serum inflammatory biomarkers and ppFEV1, differences in airway and systemic inflammation by age, gender, and lung function, intrinsic variance of serum-based proteins, and effects of freezing and delayed processing on sputum analyte measurements. To address these objectives, we examined sputum and serum inflammatory data collected from CF adolescents and adults enrolled in a multicenter clinical trial (16).
2.0. METHODS
2.1. Subjects and sample collection and processing
The original study from which these data were derived was a randomized, double-blind controlled trial of an antioxidant-enriched multivitamin supplement involving 15 U.S. CF care centers within the CF Foundation Therapeutics Development Network (CFF TDN) (ClinicalTrials.gov identifier: NCT01859390). Key inclusion/exclusion criteria and study design details were previously reported, and included subjects 10 years of age or older with confirmed CF and pancreatic insufficiency, with an ppFEV1 between 40–100, who were clinically stable for two weeks prior to randomization (16). In this clinical trial, 73 subjects were randomized, with 36 participants randomized to the active treatment group and 37 randomized to the control group. Two participants from each group withdrew from the study. For the post-hoc analyses presented in this manuscript, we used data from all 73 subjects at baseline but only used longitudinal data from the 37 subjects in the control group to avoid the potential effects the antioxidant-enriched multivitamin used in the active treatment group may have had on inflammation measurements. There were some missing data due to insufficient sample to complete all inflammation measurements. Institutional review boards at each participating center approved the study and each participant and/or their parent provided written informed consent or assent when applicable.
Fasting blood samples were collected at baseline, 4 weeks and 16 weeks. A portion was sent to each site’s clinical lab for a complete blood count, including total white blood cell (WBC) count. The remainder was processed for serum as detailed in a Study Lab Manual. Serum specimens were processed, labeled and placed immediately into a −70°C freezer locally. The frozen specimens were shipped on dry ice throughout the study to the Clinical Translational Research Center (CTRC) Core Laboratory at Children’s Hospital Colorado and University of Colorado Anschutz Medical Campus (Aurora, CO), which serves as the Center for Biochemical Markers for the CFF TDN. Induced sputum samples were obtained at baseline and 16 weeks according to a standard operating procedure. All sputum specimens were placed immediately into a −70°C freezer and shipped frozen within 24 hours to the CTRC Core Laboratory. The sputum specimens were thawed and processed upon receipt using a standard operating procedure (14, 17).
Spirometry was performed between 15 ± 5 minutes post-bronchodilator administration and before the sputum induction procedure; ppFEV1 was calculated using reference equations (18).
2.2. Inflammatory measurements
Serum aliquots were analyzed using validated commercially available assays for the following protein biomarkers: high sensitivity C reactive protein (hsCRP; Siemens Nephelometer assay, Siemens Healthcare, Tarrytown, NY), serum amyloid A (SAA; Milliplex MAP® for Luminex Technology, EMD Millipore, St. Charles, MO), calprotectin (American Laboratory Products Company-ALPCO, Salem, NH), and myeloperoxidase (MPO; ELISA, R&D systems, Minneapolis, MN). The lower limits of detection for these assays were: hsCRP, 0.007 mg/L; SAA, 500 ng/mL; calprotectin, 0.4 μg/mL; and MPO, 15 ng/mL. The intra-assay coefficients of variation (CV) were less than 15% for all assays. The sputum supernatants were analyzed for the following markers of inflammation: free neutrophil elastase activity (NE) (spectrophotometric assay based on the hydrolysis of the specific substrate MeO-suc-Ala-Ala-Pro-Ala-p-nitroanilide [Sigma Chemical Co, St. Louis, MO]), MPO (ELISA, R&D systems, Minneapolis, MN), IL-8 and TNF-α (Luminex FluorokineTechnology, R&D Systems, Minneapolis, MN). The lower limits of detection for these assays were: free neutrophil elastase activity, 8 μg/mL; MPO, 120 ng/mL; IL-8, 3, 120 pg/mL; and TNF-α, 12.5 pg/mL. All assays were performed in duplicate and mean values were used for analysis.
2.3. Pilot study of the effect of freezing and delayed processing on sputum analytes of inflammation
As preparation for the multicenter clinical trial in which sputum would undergo centralized processing and analysis in a single laboratory, we examined the effects of immediate freezing, shipping, and delayed processing on sputum analyte measurements. We consented 12 CF patients followed at Children’s Hospital Colorado under an institutionally approved specimen collection protocol and obtained spontaneously expectorated sputum from them. The samples were placed on wet ice, brought to the CTRC Core Laboratory, and manually divided into two portions using a cell scraper within 2 hours of collection. One portion underwent immediate processing using the same standard operating procedure previously referenced. The second portion was placed into a −70°C freezer. The frozen unprocessed sputum portions were shipped within 24 hours on dry ice (via FedEx, Memphis, TN, USA) to a laboratory at Seattle Children’s Hospital. This lab ensured the specimens remained frozen and then shipped them back to the CTRC Core Laboratory on dry ice. The time from sputum collection to receiving these unprocessed specimens was typically between 48–72 hours. Upon return to the lab, the specimens were thawed and underwent the exact same processing protocol as the immediately processed specimens. Supernatants from both portions were frozen at −70°C for later analysis of inflammatory markers.
2.4. Statistical Analyses
Categorical data were summarized as counts and percent (%) while means and standard deviations (SD) were reported for continuous data. All correlations reported are based on Spearman’s nonparametric rho statistic (rs), including corresponding 95% confidence intervals. A high correlation coefficient was defined as rs > 0.70 and a moderate correlation was defined as 0.50 < rs ≤ 0.70. All pairwise correlations (available-case analysis) of serum and sputum biomarkers from the baseline visit (N=73) were evaluated as well as correlations between each biomarker and ppFEV1. For each biomarker, univariate analyses using the t-test to evaluate differences in baseline concentrations by age (< 18 years versus ≥ 18 years), sex (male versus female), and ppFEV1 (< 80% versus ≥ 80%) were made. Estimates of between and within participant variability were obtained from the control arm (N=37) based on an intercept-only random effects model with participant as cluster. As this was an exploratory study meant for hypothesis generation, no formal testing or p-values were presented. Effects of freezing and delayed sputum processing on sputum inflammation measurements were evaluated using signed rank tests. Statistical analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
3.0. RESULTS
3.1. Subject characteristics
Demographics and baseline clinical characteristics of the 73 participants are shown in Table 1. Participants ranged in age from 10 to 49 years. Baseline mean ppFEV1 of this cohort was 74.1%. The sex distribution was well balanced. Most of the participants were Caucasian and were either homozygous or heterozygous for the F508del CFTR mutation, reflective of the U.S. CF population.
Table 1.
Clinical characteristics of the study participants at baseline
| All Participants (N=73) | |
|---|---|
| Sex, n (%) | |
| Female | 40 (54.8%) |
| Age (years), mean (SD) | 22.6(9.1) |
| Age distribution, n (%) | |
| >10–18 years | 29 (39.7%) |
| > 18–30 years | 28 (38.4%) |
| >30 years | 16(21.9%) |
| Race, n (%) | |
| Caucasian | 64 (87.7%) |
| Hispanic | 6 (8.2%) |
| African-American | 2 (2.7%) |
| Unknown/Other | 1 (1.4%) |
| Genotype, n (%) | |
| F508del Homozygous | 39 (53.4%) |
| F508del Heterozygous | 27 (37.0%) |
| Other/Unknown [1] | 7 (9.6%) |
| BMI (kg/m2), mean (SD) | 21.4(3.9) |
| FEV1 % Predicted [2], mean (SD) | 74.1% (14.4%) |
| FEV1 % Predicted distribution, n (%) | |
| <70% | 23(31.5%) |
| >70–<90% | 44 (60.3%) |
| >90% | 6 (8.2%) |
| Chronic Inhaled Antibiotics [3], n (%) | 40 (54.8%) |
| Chronic Azithromycin [3], n (%) | 34 (46.6%) |
Other refers to participants with either two known, non-F508del CF mutations, or one known, non-F508del CF mutation and one unidentified allele which has not been classified as a CF mutation.
FEV1 % predicted is calculated using the Global Lung Initiative reference equations.
Chronic is defined as initiated ≥8 weeks prior to randomization.
3.2. Relationships between systemic and airway inflammation measurements
Pairwise correlations for serum and sputum inflammation measurements at baseline are shown in Table 2. Several circulating biomarkers are highly correlated with one another. hsCRP and SAA correlated most strongly with each other (rs = 0.85; 95% CI: 0.77, 0.90). Calprotectin and MPO had a moderate correlation with each other (rs = 0.67; 95% CI: 0.51, 0.78). Comparing sputum-based inflammation measurements, neutrophil elastase and MPO were the most highly correlated (rs = 0.88; 95% CI: 0.82, 0.93). All the sputum biomarkers correlated well with each other including neutrophil elastase and IL-8 (rs = 0.76; 95% CI: 0.64, 0.85) and IL-8 and TNF-α (rs = 0.78; 95% CI: 0.67, 0.86). Examining the associations between serum and sputum biomarker measurements, none of the pairwise correlations exceeded the rs > 0.70 threshold. The highest correlations were between serum hsCRP and sputum neutrophil elastase, and between serum SAA and sputum neutrophil elastase (rs = 0.59 for both; 95% CIs: 0.40, 0.72 and 0.41, 0.73, respectively).
Table 2.
Spearman correlations (95% C.I.) of serum and sputum inflammatory measurements
| Serum | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| hsCRP | Calprotectin | SAA | MPO | WBC | MPO | NE | TNF−α | ||
| Serum | – | 0.55 (0.36,0.69) | 0.85 (0.77, 0.90) | 0.32 (0.09, 0.51) | 0.28 (0.05, 0.48) | ||||
| Calprotectin | – | – | 0.58 (0.40, 0.71) | 0.67 (0.51, 0.78) | 0.56 (0.38, 0.70) | 0.52 (0.32, 0.67) | 0.56 (0.37, 0.70) | 0.41 (0.19, 0.59) | |
| SAA | – | – | – | 0.29 (0.06, 0.49) | 0.38 (0.16, 0.56) | 0.49 (0.28, 0.65) | 0.59 (0.41, 0.73) | 0.43 (0.22, 0.61) | |
| MPO | – | – | – | – | 0.30 (0.07, 0.49) | 0.38 (0.16, 0.57) | 0.37 (0.15, 0.56) | 0.29 (0.06, 0.50) | |
| WBC | – | – | – | – | – | 0.46 (0.25, 0.63) | 0.45 (0.24, 0.62) | 0.41 (0.19, 0.59) | |
| Sputum | – | – | – | – | – | – | 0.88 (0.82, 0.93) | 0.69 (0.54, 0.80) | 0.73 (0.60, 0.82) |
| NE | – | – | – | – | – | – | – | 0.66 (0.50, 0.77) | 0.76 (0.64, 0.85) |
| TNF-α | – | – | – | – | – | – | – | – | 0.78 (0.67, 0.86) |
| – | – | – | – | ||||||
Note: Shaded areas represent correlations that were either moderate or high.
3.3. Relationships between inflammation measurements and lung function
Correlations between baseline inflammation measurements in serum and sputum and baseline ppFEV1 are shown in Table 3. The biomarkers that correlated most strongly with ppFEV1 were serum hsCRP (rs = −0.55; 95% CI: −0.69, −0.37), serum SAA (rs = −0.58; 95% CI: −0.72, −0.41), and sputum neutrophil elastase (rs = −0.53; 95% CI: −0.68, −0.34) (Figure 1 A–C). Longitudinal associations were examined using the control cohort for whom measurements were available at baseline and the 16-week time point. There were a few significant associations that showed an inverse relationship between changes in biomarkers and ppFEV1 over 16 weeks (Table 3). The change in biomarkers which correlated most strongly with change in ppFEV1 were serum hsCRP and sputum neutrophil elastase (rs = −0.42 for both; 95% CIs: −0.66, −0.10 and −0.69, −0.06, respectively Figure 1 D–F). To determine whether a short-term change in a serum biomarker was associated with a longer-term change in ppFEV1, we examined correlations between the 4 week change in serum biomarkers and the 4 and 16 week changes in ppFEV1 (Supplemental Table 1). Changes in circulating WBC and hsCRP over 4 weeks correlated with 4-week changes in ppFEV1, while only the 4-week change in WBC correlated with the 16-week change in ppFEV1.
Table 3.
Correlations between inflammation measurements in serum and sputum and ppFEV1 (baseline and 16 week change from baseline)
| Baseline [1] | 16 Week Change [2] | |||||
|---|---|---|---|---|---|---|
| Analytes | N | Spearman’s correlation | 95% CI | n | Spearman’s correlation | 95% CI |
| WBC | 73 | −0.32 | (−0.51,−0.09) | 34 | −0.38 | (−0.64, −0.05) |
| hs-CRP | 72 | −0.55 | (−0.69, −0.37) | 35 | −0.42 | (−0.66,−0.10) |
| Calprotectin | 72 | −0.48 | (−0.64, −0.28) | 35 | −0.33 | (−0.60, 0.01) |
| SAA | 72 | −0.58 | (−0.72,−0.41) | 35 | −0.34 | (−0.60, 0.00) |
| MPO | 72 | −0.23 | (−0.44, 0.00) | 35 | −0.25 | (−0.54, 0.09) |
| Sputum MPO | 69 | −0.44 | (−0.62, −0.23) | 28 | −0.30 | (−0.60, 0.08) |
| NE | 69 | −0.53 | (−0.68, −0.34) | 28 | −0.42 | (−0.69, −0.06) |
| TNF-alpha | 69 | −0.41 | (−0.59,−0.19) | 28 | −0.33 | (−0.62, 0.05) |
| IL-8 | 69 | −0.39 | (−0.57,−0.17) | 28 | −0.22 | (−0.55, 0.17) |
Correlation between baseline biomarker and baseline ppFEV1 from total cohort (N=73)
Correlation between 16 week change in biomarker and 16 week change in ppFEV1 from control cohort (N=37)
Figure 1. Correlations between biomarkers (hs-CRP, SAA and HNE) and lung function at baseline (panels A-C) and 16-week change (panels D-F).
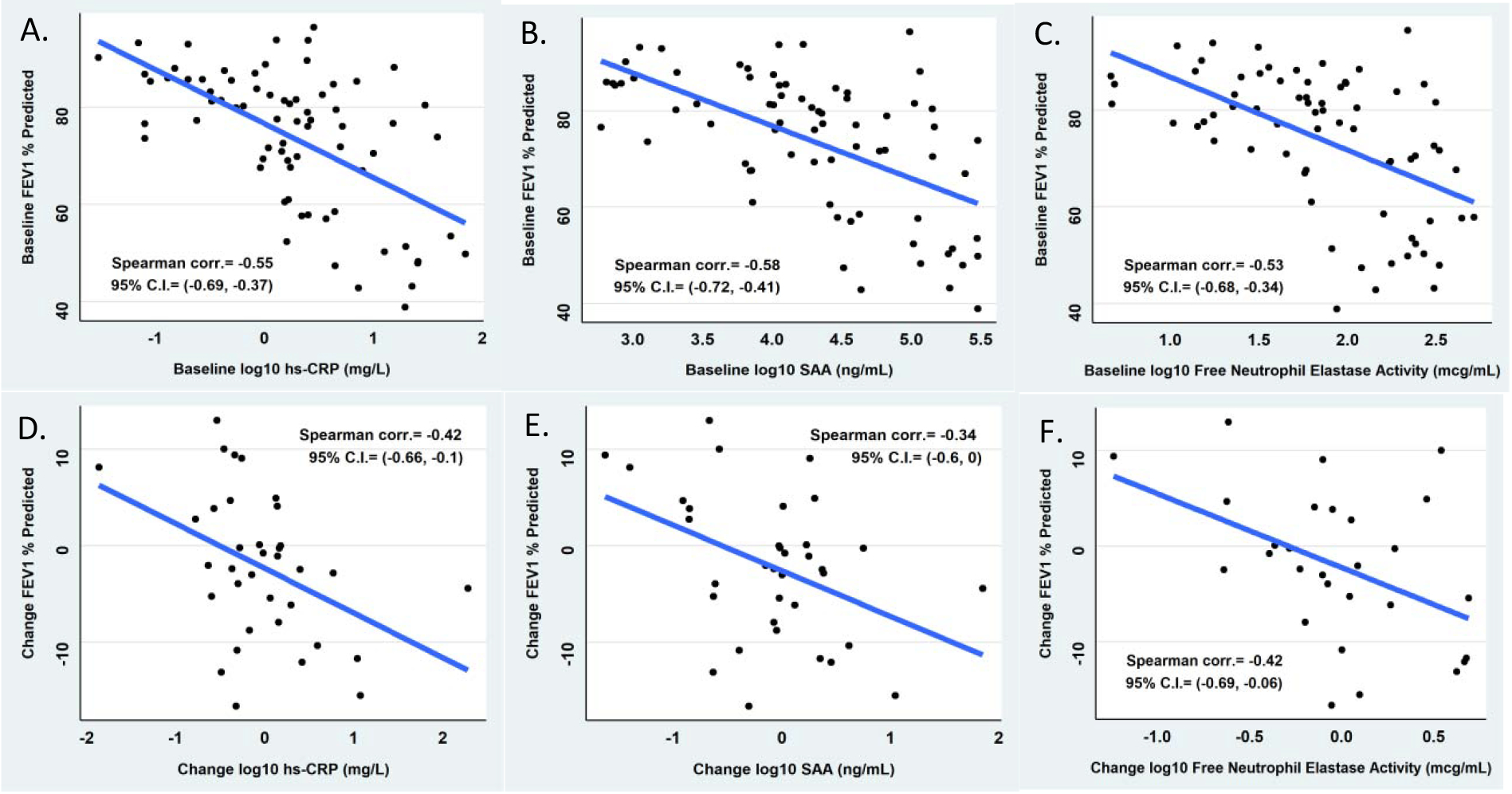
Correlations between biomarker and lung function at baseline in the entire study cohort (n = 73) and correlations between changes in biomarker and lung function over 16 weeks in the control cohort (n = 37).
3.4. Differences in systemic and airway inflammation by age, gender, and lung function
Serum hsCRP and MPO and sputum neutrophil elastase, TNF-α, IL-8, and MPO were significantly higher in adults (≥ 18 years) compared with children and adolescents (< 18 years) (Supplemental Table 2). There were no statistically significant differences in any of the biomarkers between males and females. All of the serum and sputum biomarkers were significantly higher in those with lower ppFEV1 (< 80%) compared with those with more preserved ppFEV1 (> 80%). While supplemental Table 2 shows differences between age groups (< 18 versus ≥ 18 years) in terms of inflammatory markers, we further show that even within a particular age group, many biomarkers are inversely related to ppFEV1, indicating that both of the predictors, age and ppFEV1, affect biomarker concentrations (Supplemental Table 3).
3.5. Variability of serum inflammation measurements
Variability of the serum biomarker measurements were determined for the participants in the control cohort who had two collections separated by four weeks. The within-subject standard deviation was lower than between-subject standard deviation for each of the serum biomarkers (Supplemental Table 4). The rank order of observed within-subject variability for each of the serum biomarkers (lowest to highest) was calprotectin, MPO, hsCRP, SAA, and circulating WBC. The serum biomarkers with the highest ratio of between-to-within subject variability were MPO and calprotectin.
3.6. Effects of freezing and delayed processing on sputum inflammation measurements
There were no significant differences in paired measurements of neutrophil elastase, IL-1β, and MPO in the sputum aliquots that underwent immediate processing (‘fresh’) compared to the sputum aliquots that underwent delayed processing following shipping (‘frozen’), though neutrophil elastase trended higher in the fresh sputum aliquots versus the paired frozen aliquots (Supplemental Figure 1).
4.0. DISCUSSION
In this multicenter cohort of adolescents and adults with CF, we measured several biomarkers of inflammation in blood and sputum to find informative biomarkers, which we believe can be used investigate treatment effects of anti-inflammatory compounds for CF. The large cohort of over 70 subjects allowed us to explore interrelationships between systemic and airway inflammation and to determine associations between inflammatory biomarkers and ppFEV1 in a more heterogeneous CF population than has been typically derived from single center cohorts. The collection of serum samples twice within 4 weeks allowed us to assess the reproducibility of circulating biomarkers. Additionally, we performed a pilot study to examine the effects of immediate freezing, shipping, and delayed processing on sputum analyte measurements. The results of this investigation will help to select markers for CF anti-inflammatory clinical trials.
In terms of the interrelationships between systemic and airway inflammation, several biomarkers of inflammation in blood and sputum correlated with one another. Among circulating biomarkers, serum hsCRP and SAA correlated most strongly with each other, while sputum neutrophil elastase and MPO were the most highly correlated among airway inflammatory measures. Based on these data, it would be redundant to measure serum hsCRP and SAA together and sputum neutrophil elastase and MPO together. It may be better to assess unrelated (orthogonal) biomarkers that reflect different, independent biologic inflammatory pathways. For example, there may be justification to measuring serum calprotectin, a marker of neutrophilic inflammation, along with serum hsCRP, a non-specific marker of systemic inflammation. Correlations across matrices between serum and sputum biomarker measurements were not as strong as correlations within the same matrix. This suggests that systemic and pulmonary inflammation in CF may arise because of distinct mechanisms (e.g. different stimuli and biologic pathways) and that systemic inflammation, which may reflect important comorbidities including CF-related diabetes, digestive and liver disease, and bone disease, is not solely due to “spill over” of mediators from the lung.
Lung function is an important indicator of disease severity, though it may not be as reflective of disease activity in the lungs. Biomarkers would be clinically useful and provide added value to lung function testing if they could discriminate disease activity among patients with the same disease severity. Also, an important step in the validation process of biomarkers as outcome measures in clinical trials assessing early biologic efficacy of anti-inflammatory and antimicrobial agents is to establish that they are correlates of established clinical outcome measures including lung function. Examining relationships between inflammation measurements and lung function, the biomarkers that correlated most strongly with ppFEV1 at a single time point were serum hsCRP, SAA, and sputum neutrophil elastase. Interestingly, circulating hsCRP and SAA correlated just as strongly with ppFEV1 as did a local measure of pulmonary inflammation, sputum neutrophil elastase. Further, 16-week changes in serum hsCRP and sputum neutrophil elastase correlated to a similar extent with changes in ppFEV1. These biomarkers may serve as correlates of an important objective measurement of lung disease severity in CF. We did not explore whether a composite measurement of both systemic and airway inflammation (i.e. combining serum and sputum biomarkers) is more strongly associated with ppFEV1 than are the individual biomarkers in blood and sputum.
Most of the serum and sputum biomarkers were significantly higher in adults and those with lower ppFEV1 (< 80%) compared with adolescents and those with more preserved ppFEV1 (> 80%). In stratified analyses, both age and underlying lung function were shown to influence biomarker concentrations. This is an important consideration in clinical trials if discrepant anti-inflammatory effects are observed in these patient populations. For instance, differences in baseline inflammation might explain divergent response to therapy in adults and those with lower lung function compared with children and those with more preserved lung function.
Our analysis adds to the limited studies that have examined biomarkers in blood and sputum simultaneously in CF (10–12, 19). Most of these studies were single center and mainly enrolled adults with CF. The study that was most similar to ours measured sputum and serum inflammatory markers in 44 pediatric and adult patients with CF across 3 CF centers during and after a pulmonary exacerbation (12). In their cohort, several biomarkers of inflammation in blood and sputum correlated with one another within the same matrix whereas most biomarkers were uncorrelated across matrices and only one biomarker, serum IL-8, correlated with ppFEV1. While the average age and age distribution of their cohort were like our subjects, our study included a larger number and we studied subjects during a time of clinical stability rather than during pulmonary exacerbations.
In the control cohort who underwent serum collection twice within a 4-week period, we showed reasonably good reproducibility of several serum biomarkers of inflammation, particularly calprotectin, MPO, and hsCRP. The inter- and intra-patient variability of serum inflammatory markers are less than those observed in sputum (17). In theory, this would mean that fewer subjects would be required in CF clinical trials of anti-inflammatory drugs which rely upon serum inflammatory biomarkers as endpoints compared with sputum inflammatory markers. However, this would not hold true if the potential therapeutic had less of an impact on systemic inflammation than on pulmonary inflammation. Also, while low intra-subject variability is a desirable biomarker characteristic, the magnitude of change of the biomarker in response to a beneficial therapy would still need to be large enough to overcome this variability. Even though fasting blood samples were used in this study, a recent publication suggests that the timing of food intake does not preclude being able to identify differences in concentrations of circulating inflammatory proteins between cohorts, supporting the use of non-fasting blood samples for inflammatory marker measurements in clinical trials (20).
A key consideration with respect to analyzing sputum biomarkers of inflammation in multicenter clinical trials is whether sputum can be frozen immediately following collection and undergo delayed processing (homogenization) in a centralized laboratory. The need for specialized laboratories and trained technologists to process sputum samples is a significant barrier to incorporating the routine collection of sputum in multicenter CF clinical trials. Importantly, examination of sputum cytology (cellularity) is limited by the need to process samples within 2 hours of collection (21, 22). However, the effects of freezing and delayed processing on biochemical mediators in sputum including proteases and cytokines is less clear. One publication found that storage at −70°C prevented the degradation of sputum (23). Another publication reported that there were no significant effects of freezing and thawing on measurements of IL-8 and TNF-α in sputum supernatant that had already undergone partial homogenization and centrifugation to remove host cells (24). In contrast, overnight shipping at 4°C and delayed processing appeared to influence HMGB-1 measurements in a small number (n=7) of sputum specimens (25). In our pilot investigation, there were no significant differences in paired measurements of neutrophil elastase, IL-1β, and MPO in the sputum aliquots that underwent immediate processing compared to the sputum aliquots that underwent delayed processing following freezing and shipping, though neutrophil elastase trended higher in the immediately processed sputum aliquots. This requires further investigation in a larger number of sputum samples. If it is confirmed that freezing and delayed processing do not significantly affect common sputum analytes including neutrophil elastase activity, this will be particularly relevant to multicenter trials allowing for sputum samples to be collected, frozen, and shipped to centralized sputum processing laboratories.
The main limitation of this study is that it is a post-hoc analysis of data collected in an interventional trial. However, this clinical trial provided the opportunity to analyze data from a large heterogenous group of adolescents and adults with CF. It was not designed or powered specifically to determine the most reliable and informative biomarkers for CF clinical trials. Since sputum was not collected at the 4-week time point, we could not directly compare the reproducibility of sputum biomarkers with serum biomarkers at this time. As previously mentioned, we analyzed a small number of sputum samples in the pilot study examining the effects of freezing and delayed processing on sputum analytes of inflammation, which requires further investigation in a larger number of samples.
In summary, sputum neutrophil elastase and serum hsCRP and SAA correlate to a similar extent with ppFEV1 at a single time point. Changes in serum hsCRP and sputum neutrophil elastase are comparably associated with changes in ppFEV1 over 16 weeks. Since serum hsCRP correlates strongly with SAA, simply measuring one of these biomarkers of systemic inflammation would be sufficient. Based on the biomarkers analyzed in this study, serum hsCRP and sputum neutrophil elastase are promising candidates to include in CF anti-inflammatory clinical trials to avoid redundancy, minimize variation, and serve as correlates of lung disease severity and change, but further studies designed to specifically answer these questions are needed to validate these results and make more wide scale conclusions.
Supplementary Material
Highlights:
Safe and effective anti-inflammatory drugs are needed for people with CF.
Sputum and serum inflammatory biomarkers can aid CF drug development.
Some biomarkers of inflammation correlate with each other.
Biomarkers of inflammation correlate with lung function.
Measurements of serum inflammatory biomarkers are reproducible over 4 weeks.
5.0. ACKNOWLEDGMENTS
Rebecca Baldermann and the lab technicians in the CTRC Core Laboratory for processing the biospecimens and performing the measurements of inflammation; CFF TDN Coordinating Center for oversight of the clinical trial; all of the study participants and their families.
Sources of support: This research was supported by Cystic Fibrosis Foundation Therapeutics (AQUADEK12K1), and by NIH/NCATS Colorado CTSA #UL1 TR002535.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trial registration available at www.clinicaltrials.gov, ID NCT01859390.
CONFLICTS OF INTEREST:The authors report the following conflicts of interest relevant to this manuscript. A.B., R.J., U.K., and S.S. received grant support from the Cystic Fibrosis Foundation related to this project.
REFERENCES
- 1.Nichols DP, Chmiel JF. Inflammation and its genesis in cystic fibrosis. Pediatr Pulmonol. 2015;50 Suppl 40:S39–56. [DOI] [PubMed] [Google Scholar]
- 2.Eigen H, Rosenstein BJ, FitzSimmons S, Schidlow DV. A multicenter study of alternate-day prednisone therapy in patients with cystic fibrosis. Cystic Fibrosis Foundation Prednisone Trial Group. J Pediatr. 1995;126(4):515–23. [DOI] [PubMed] [Google Scholar]
- 3.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med. 1995;332(13):848–54. [DOI] [PubMed] [Google Scholar]
- 4.Lands LC, Milner R, Cantin AM, Manson D, Corey M. High-dose ibuprofen in cystic fibrosis: Canadian safety and effectiveness trial. J Pediatr. 2007;151(3):249–54. [DOI] [PubMed] [Google Scholar]
- 5.Oermann CM, Sockrider MM, Konstan MW. The use of anti-inflammatory medications in cystic fibrosis: trends and physician attitudes. Chest. 1999;115(4):1053–8. [DOI] [PubMed] [Google Scholar]
- 6.Lai HC, FitzSimmons SC, Allen DB, Kosorok MR, Rosenstein BJ, Campbell PW, et al. Risk of persistent growth impairment after alternate-day prednisone treatment in children with cystic fibrosis. N Engl J Med. 2000;342(12):851–9. [DOI] [PubMed] [Google Scholar]
- 7.Konstan MW, VanDevanter DR, Rasouliyan L, Pasta DJ, Yegin A, Morgan WJ, et al. Trends in the use of routine therapies in cystic fibrosis: 1995–2005. Pediatr Pulmonol. 2010;45(12):1167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris JK, Wagner BD, Zemanick ET, Robertson CE, Stevens MJ, Heltshe SL, et al. Changes in Airway Microbiome and Inflammation with Ivacaftor Treatment in Patients with Cystic Fibrosis and the G551D Mutation. Ann Am Thorac Soc. 2020;17(2):212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torphy TJ, Allen J, Cantin AM, Konstan MW, Accurso FJ, Joseloff E, et al. Considerations for the Conduct of Clinical Trials with Antiinflammatory Agents in Cystic Fibrosis. A Cystic Fibrosis Foundation Workshop Report. Ann Am Thorac Soc. 2015;12(9):1398–406. [DOI] [PubMed] [Google Scholar]
- 10.Gray RD, Imrie M, Boyd AC, Porteous D, Innes JA, Greening AP. Sputum and serum calprotectin are useful biomarkers during CF exacerbation. J Cyst Fibros. 2010;9(3):193–8. [DOI] [PubMed] [Google Scholar]
- 11.Martin SL, Moffitt KL, McDowell A, Greenan C, Bright-Thomas RJ, Jones AM, et al. Association of airway cathepsin B and S with inflammation in cystic fibrosis. Pediatr Pulmonol. 2010;45(9):860–8. [DOI] [PubMed] [Google Scholar]
- 12.Horsley AR, Davies JC, Gray RD, Macleod KA, Donovan J, Aziz ZA, et al. Changes in physiological, functional and structural markers of cystic fibrosis lung disease with treatment of a pulmonary exacerbation. Thorax. 2013;68(6):532–9. [DOI] [PubMed] [Google Scholar]
- 13.Sagel SD, Sontag MK, Wagener JS, Kapsner RK, Osberg I, Accurso FJ. Induced sputum inflammatory measures correlate with lung function in children with cystic fibrosis. J Pediatr. 2002;141(6):811–7. [DOI] [PubMed] [Google Scholar]
- 14.Sagel SD, Wagner BD, Anthony MM, Emmett P, Zemanick ET. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2012;186(9):857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liou TG, Adler FR, Keogh RH, Li Y, Jensen JL, Walsh W, et al. Sputum biomarkers and the prediction of clinical outcomes in patients with cystic fibrosis. PLoS One. 2012;7(8):e42748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagel SD, Khan U, Jain R, Graff G, Daines CL, Dunitz JM, et al. Effects of an Antioxidant-enriched Multivitamin in Cystic Fibrosis. A Randomized, Controlled, Multicenter Clinical Trial. Am J Respir Crit Care Med. 2018;198(5):639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chmiel JF, Konstan MW, Accurso FJ, Lymp J, Mayer-Hamblett N, VanDevanter DR, et al. Use of ibuprofen to assess inflammatory biomarkers in induced sputum: Implications for clinical trials in cystic fibrosis. J Cyst Fibros. 2015;14(6):720–6. [DOI] [PubMed] [Google Scholar]
- 18.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downey DG, Martin SL, Dempster M, Moore JE, Keogan MT, Starcher B, et al. The relationship of clinical and inflammatory markers to outcome in stable patients with cystic fibrosis. Pediatr Pulmonol. 2007;42(3):216–20. [DOI] [PubMed] [Google Scholar]
- 20.Sagel SD, Wagner BD, Ziady A, Kelley T, Clancy JP, Narvaez-Rivas M, et al. Utilizing centralized biorepository samples for biomarkers of cystic fibrosis lung disease severity. J Cyst Fibros. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kips JC, Fahy JV, Hargreave FE, Ind PW, in’t Veen JC. Methods for sputum induction and analysis of induced sputum: a method for assessing airway inflammation in asthma. Eur Respir J Suppl. 1998;26:9S–12S. [PubMed] [Google Scholar]
- 22.Efthimiadis A, Spanevello A, Hamid Q, Kelly MM, Linden M, Louis R, et al. Methods of sputum processing for cell counts, immunocytochemistry and in situ hybridisation. Eur Respir J Suppl. 2002;37:19s–23s. [DOI] [PubMed] [Google Scholar]
- 23.Charman J, Reid L. The effect of freezing, storing and thawing on the viscosity of sputum. Biorheology. 1973;10(3):295–301. [DOI] [PubMed] [Google Scholar]
- 24.Tirelli AS, Colombo C, Torresani E, Cariani L, Arnaboldi E, Conese M. Validation of an automated sensitive immunoassay for quantitation of cytokines in the sputum of cystic fibrosis patients. Clin Chem Lab Med. 2007;45(1):108–11. [DOI] [PubMed] [Google Scholar]
- 25.Liou TG, Adler FR, Argel N, Asfour F, Brown PS, Chatfield BA, et al. Prospective multicenter randomized patient recruitment and sample collection to enable future measurements of sputum biomarkers of inflammation in an observational study of cystic fibrosis. BMC Med Res Methodol. 2019;19(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


