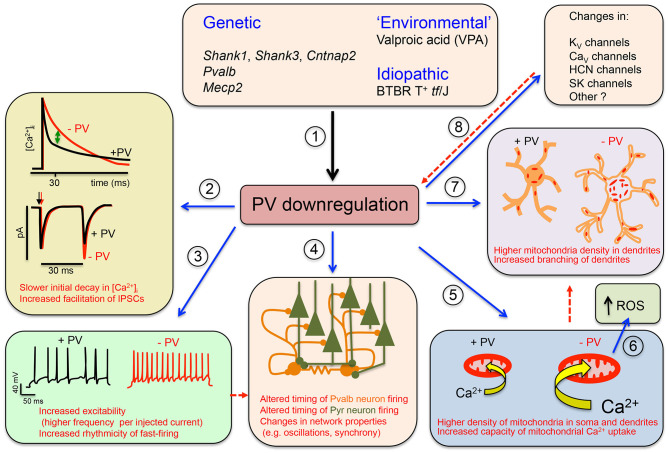
Figure 1.
Graphical summary of the Parvalbumin Hypothesis of autism spectrum disorder (ASD) (and putatively, of schizophrenia). (1) Genetic and environmental factors directly or indirectly affecting Pvalb neuron function in mice through impairment of principal neurons, leading to the downregulation of parvalbumin (PV) and reduced neuronal function. (2) (Upper) The initial decline in [Ca2+]i in Pvalb neurons is accelerated in the presence of PV. Differences in the decay curves (±PV) are largest in the time window of ~20–50 ms after peak [Ca2+]i, resulting in increased residual Ca2+ in the absence of PV (green arrow). (Lower) Larger amounts of residual Ca2+ promote γ-aminobutyric acid (GABA) release during further stimulation in this time window, leading to increased facilitation in the paired-pulse protocol. (3) An absence of PV alters other electrophysiological properties: for example, it may increase the excitability of Pvalb neurons, or may result in more regular firing within AP trains (i.e., reducing “jitter”) (modified from Orduz et al., 2013). (4) A decrease in PV level affects the electrophysiological properties not only of Pvalb neurons but also of principal (pyramidal) cells and modifies network properties such as oscillation and synchrony. (5) A decrease in PV level alters Ca2+-dependent excitation/transcription coupling by reducing transcript levels of activity-driven genes including Pvalb and Gad1 and prototypical neuronal-activity-associated genes such as c-Fos, Arc, and Wnt2; it also increases messenger RNA (mRNA) levels of genes involved in mitochondrial biogenesis such as Ppargc1a encoding the mitochondria master regulator PGC-1α, as well as Nrf1 and Tfam. This results in an increase in mitochondrial volume (density) that is approximately proportional to the PV concentration in corresponding wild-type (WT) Pvalb neurons—i.e., the higher the PV concentration, the greater the increase in mitochondrial density in PV−/− Pvalb neurons. (6) Increased mitochondrial volume associated with a decrease in PV level enhances mitochondrial Ca2+-buffering/sequestration capacity, thereby promoting reactive oxygen species (ROS) production. This slow process may impair mitochondrial function and ultimately impacts Pvalb neuron function, which is thought to produce a schizophrenia phenotype. (7) Absence of PV during (early) postnatal neurodevelopment from ~PND7 to PND20 increases dendritic branching, yielding Pvalb neurons with a larger dendritic tree and possibly resulting in hyperconnectivity. Increases in mitochondria number and dendritic branching caused by PV downregulation may be subsequent events (red dashed arrow) or could occur via independent mechanisms. (8) Absence of PV perturbs the expression of other putative ASD susceptibility genes that are either directly associated with the electrophysiological properties of Pvalb neurons or are expressed in pyramidal cells that influence Pvalb neuron circuitry; this includes KV, CaV HCN channels, and SK channels. Whether targeted inhibition of these genes also affects PV level is unknown. Solid arrows indicate a causal relationship between events—e.g., altered Ca2+ concentration (±PV) and short-term modulation of synaptic plasticity. Dashed lines indicate putative (indirect) mechanism(s) via as yet unknown pathways. Points (1)–(8) are described in detail in the main text (see all subsections in Data Supporting the Parvalbumin Hypothesis of ASD).