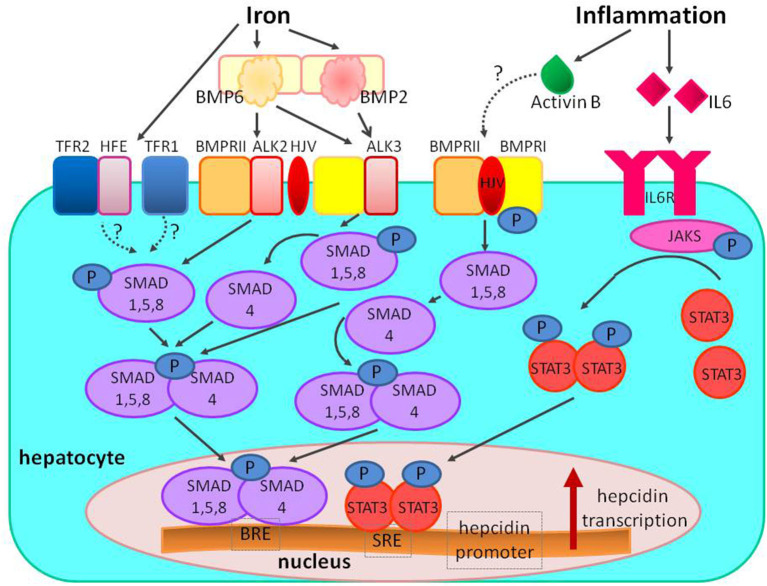
Figure 5.
The regulation of hepcidin expression by iron and inflammation. Increased systemic iron stimulates the production of the ligand BMP6, which binds to the BMPRI (ALK2/ALK3) and BMPRII receptors, and the co-receptor HJV to stimulate phosphorylation of the SMAD1/5/8 signaling molecules; phosphorylated SMAD1/5/8 binds to SMAD4 and translocates to the nucleus to bind BRE in the hepcidin promoter. In iron overload diferric transferrin displaces HFE from TFR1 to enable iron uptake and stabilizes TFR2 potentiating SMAD signaling (the role of the other HFE and TFR2 proteins is still unclear). Inflammation also stimulates hepcidin production; inflammation increases IL-6 binding to IL-6R and stimulating phosphorylation of JAKs and STAT3; phosphorylated STAT3 homodimers translocate to nucleus and bind to SRE in hepcidin promoter. The proposed mechanism of interaction between inflammatory and BMP signaling includes activin B, which is induced by inflammation, and binds to BMPRs to stimulate SMAD1/5/8 phosphorylation. The interaction between SMAD and STAT can also take place at the level of hepcidin promoter. BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor; SMAD, suppressor of mothers against decapentaplegic proteins; JAK, Janus kinase; STAT3, signal transducer and activator of transcription; HJV, hemojuvelin; HFE, hemochromatosis protein; IL-6, interleukin 6; IL-6R, interleukin 6 receptor; TfR, transferrin receptor; BRE, BMP-responsive elements in the hepcidin promoter; SRE, STAT-responsive elements in the hepcidin promoter; P, phosphate.