Figure 3 |. Hepcidin regulation.
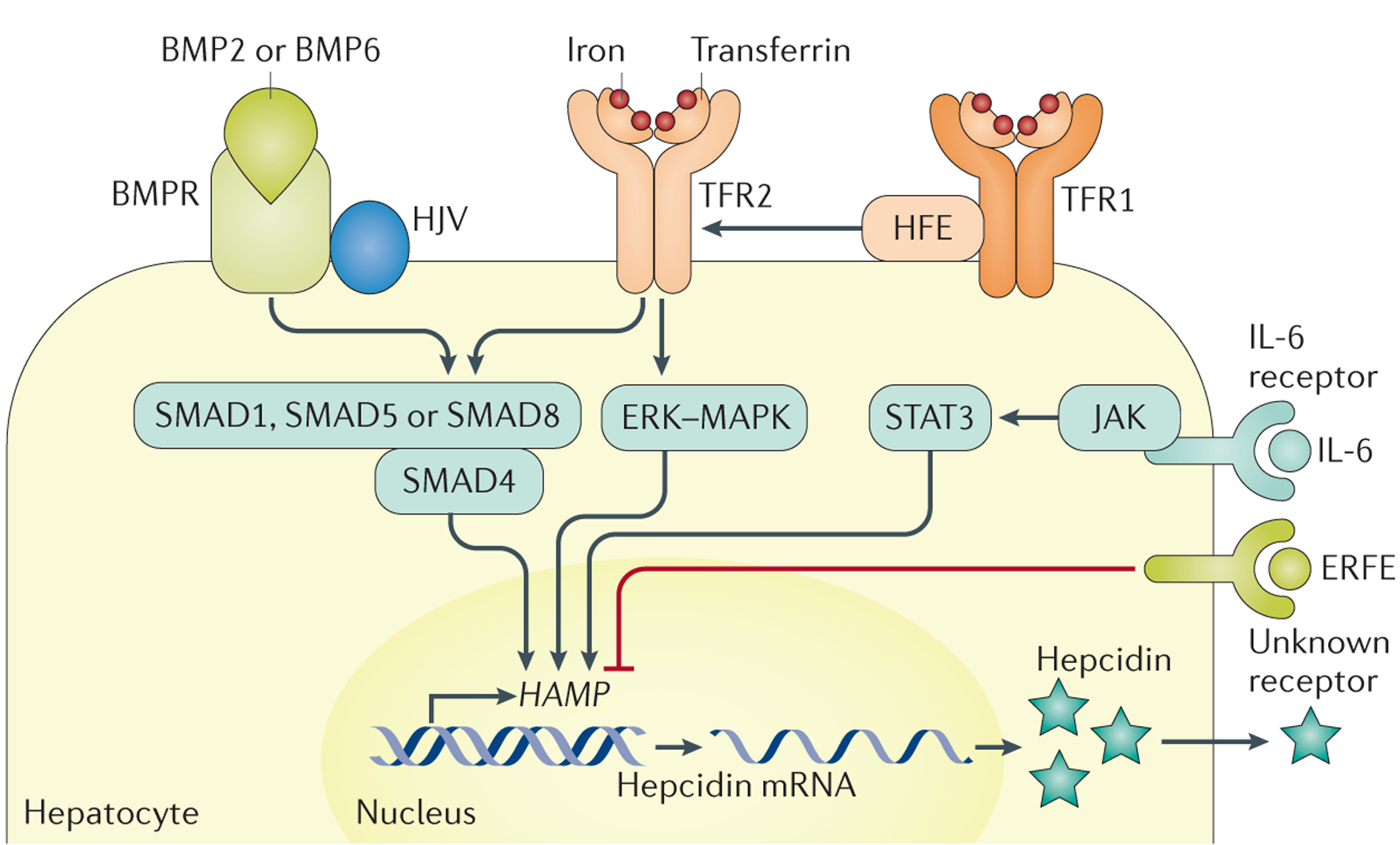
Increased cell iron stores lead to increased bone morphogenetic protein 6 (BMP6) expression in liver cells (sinusoidal cells are probably the major producers217,218, but hepatocytes and stellate cells might also be involved). BMP6 binds to the heterodimeric BMP receptor (BMPR) type 1 and type 2, which are bound to haemojuvelin (HJV)219,220. BMP-BMPR binding leads to BMPR phosphorylation, which leads to the phosphorylation of mothers against decapentaplegic homologue 1 (SMAD1), SMAD5 or SMAD8 (REF. 221). These proteins form a complex with SMAD4, which is transported into the nucleus and interacts with BMP-responsive elements on the HAMP promoter, leading to HAMP transcription and hepcidin expression102,222–224. Transferrin saturation is also involved in hepcidin regulation. This process could involve a shift of the interactions between hereditary haemochromatosis protein (HFE), transferrin receptor 2 (TFR2) and transferrin receptor 1 (TFR1) with increased transferrin saturation190, leading to signalling — potentially through mitogen-activated protein kinase (MAPK) and extracellular-signal-regulated kinase (ERK)225 — to increase HAMP transcription, although this process is not fully understood. The HFE-TFR1-TFR2 complex could also interact with the BMPR-HJV complex63. Other regulators of hepcidin expression include chronic inflammation, which is mediated by IL-6 produced by inflammatory cells and induces Janus kinase (JAK) and signal transducers and activators of transcription 3 (STAT3) activation68–71, in addition to erythroferrone (ERFE), which is produced by erythroblasts and interacts with unknown partners to decrease HAMP transcription. Figure adapted from REF. 216, Macmillan Publishers Limited.
